Design and progress of a factorial trial testing the effect of spironolactone and inorganic nitrate on arterial function in people at risk of or with type 2 diabetes
- DOI
- 10.1016/j.artres.2015.10.194How to use a DOI?
- Keywords
- Arterial stiffness; Pulse wave velocity (PWV); Cardio-ankle vascular index (CAVI); Spironolactone; Beetroot; Nitrate
- Abstract
Background: Arterial stiffness (AS), as pulse wave velocity (PWV), is a powerful independent predictor of cardiovascular events and commonly complicates type 2 diabetes (T2D). This trial aims to test if AS, measured by the VaSera machine as cardio-ankle vascular index (CAVI) and by Arteriograph measuring central PWV, can be reduced by spironolactone and/or inorganic nitrate from beetroot juice independently of blood pressure (BP) in those with or at risk of T2D.
Methods: A factorial design, double blind, randomised controlled trial in 18–80 year old men and women clinically diagnosed with T2D or at risk of it (body mass index (BMI) ≥ 27 kg m−2, positive family history or glucose intolerance). The study lasts up to 36 weeks with daily intervention of either ≤50 mg spironolactone (intervention) or ≤16 mg doxazosin (control), and beetroot juice with ≤400 mg (9 mmol) inorganic nitrate (intervention) or placebo beetroot juice, 0 mg nitrate (control). Non-invasive AS measurements are carried out at baseline and then at 12-week intervals thereafter.
Results: To date, 95 participants have been consented and screened, 19 of these were not suitable or not willing to participate so that 73 have been randomised with 9 participants screened as eligible and awaiting randomisation. 53 participants have completed the study. Mean baseline and follow up measures of cardiac-ankle and cardiac-aortic bifurcation PWV and BP have been straightforward.
Conclusion: This is a proof-of-principle trial to alter AS independent of BP in a patient sample at high cardiovascular risk.
Clinical trial registration information: UK Clinical Research Network Portfolio Database: 25003627.
- Copyright
- © 2015 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Background
Type 2 diabetes mellitus (T2D) is a chronic disease, possibly due to excess fat accumulation with inflammatory damage to blood vessels, currently diagnosed with elevated blood glucose. The rapid increase in T2D worldwide is due to both the obesity epidemic and the ageing population.1 Cardiovascular complications of T2D tend to emerge relatively early, with 54% of deaths in T2D patients being attributed to cardiovascular disease.2 In these patients, deposition of collagen matrixes in the media of blood vessels is thought to cause arterial stiffening.3
Aldosterone, the mineralocorticoid steroid hormone produced by the adrenal gland increasing salt and water retention, is involved in blood pressure (BP) regulation. Increasing evidence suggests that aldosterone causes collagen deposition in vascular walls,4,5 hence promoting arterial stiffening. Spironolactone is a mineralocorticoid receptor antagonist used primarily as a diuretic, but noted for its ability to control BP, although not formally licensed for this indication.6 Although its BP lowering effects are established, the drug has also been shown to reduce arterial stiffening, measured as pulse wave velocity (PWV) in hypertensive diabetic patients in a small study (n = 10); however, how far this PWV effect was related to BP reduction is unclear.7 A longer term study reported that spironolactone at 25 mg/day for 40 weeks reduced PWV independently of BP, although the latter still fell by 11 ± 12 mmHg, in addition to a reduction in left ventricular mass.8
Improving vascular function by dietary means is becoming increasingly appealing for the public and clinicians alike. Foods rich in inorganic nitrate, such as spinach and beetroot have been shown to exhibit beneficial effects on the vascular system such as improving endothelial dysfunction,11 inhibiting platelet aggregation9 and adhesion10 in the endothelium and improving endothelial dysfunction.11 Further, nitrates are becoming an established means of reducing BP, as found in short term randomised controlled trials (RCTs).12–15 Nitrate containing foods are thought to work by uptake of their naturally occurring nitrate into the entero-salivary circulation and subsequent conversion via the nitrate–nitrite–nitric oxide (NO) pathway, with NO, the ubiquitous vasodilator from endothelium, mediating the reduction in BP. Less work, however, has investigated the effect of dietary nitrate on arterial stiffening; an acute investigation using a high nitrate spinach meal did not alter PWV in healthy volunteers,16 however a study in drug naive grade one hypertensive patients did show a reduction in PWV after beetroot juice ingestion.17
PWV has long been an established technique of assessing arterial stiffness. While applanation tonometry has been the most widely used method to measure what is known as carotid-femoral (cf) PWV,18 as those vessel sites are used for measurement, other less intrusive means are now available. These cuff based measurements, Arteriograph and Mobil-o-graph (which measure central or aortic (ao) PWV) are becoming increasingly popular due to ease of application, the reduced amount of training required, comfort for patients and seemingly comparing well against other methods, including magnetic resonance (MR), once arterial path length is calibrated.19 An alternative device, the VaSera machine (by Fukuda Denshi) measures cardio-ankle PWV, expressed as the cardio-ankle vascular index (CAVI) to reduce dependence from ambient BP.20
Here, we describe the design of our VaSera trial, assessing the efficacy of spironolactone and dietary nitrate (as beetroot juice) to alter arterial stiffness, primarily as measured by CAVI, independently of BP.
Study design and methods
Overall design
The objective is to test, in a factorial designed RCT, whether spironolactone, up to 50 mg/day and/or dietary nitrate, up to 9 mmol nitrate per day as a 70 mL shot of ‘Beet-it’ or ‘Beet-it Sport’ beetroot juice, (compared with up to 16 mg/day doxazosin and identical 70 mL nitrate free juice respectively) reduces arterial stiffness after up to 6 months, measured as CAVI by the VaSera machine and as aoPWV by the Arteriograph, in patients diagnosed with or at risk of T2D (see Table 1).
| Nitrate juice (4.5–9 mmol nitrate/day) | Placebo juice (0 mmol nitrate/day) | Total (n) | |
|---|---|---|---|
| Spironolactone (12.5–50 mg/day) | 25–30 | 25–30 | 50–60 |
| Doxazosin (4–16 mg/day) | 25–30 | 25–30 | 50–60 |
| Total (n) | 50–60 | 50–60 | 100–120 |
Factorial design of the VaSera trial, with number of participants to be randomised to each arm.
After the initial consenting and screening/familiarisation visit, visit 1 (V1), if the criteria were met (as below), patients were invited to return for a randomisation visit, visit 2 (V2), to be allocated to one of the four groups (Table 1). Participants and those running the study are blinded to the intervention allocation, which was block randomised in groups of six, performed and held by an independent party. At V2 baseline vascular measurements (CAVI, PWV and echocardiogram) are performed, in addition to baseline blood, spot urine and 24 h urine collection for various biochemical assessments (HbA1c, glucose, renal function as well as a urinary steroid metabolite profile); 24 h BP and PWV monitoring is also performed before the intervention is started. Study medication and juice is given at this visit; titrated up to 25 mg/day spironolactone or 4 mg/day doxazosin and beetroot juice with 4.5 mmol nitrate/day or control 0 mmol nitrate/day. A check up visit for renal function and well being occurs 2 weeks into the study (V3), then again with PWV and BP at 8 weeks after randomisation (V4). At 12 weeks post randomisation (V5) a further visit with vascular measures and detailed biochemical assessment and repeat 24 h monitoring is carried out as at V2. At this visit, the medication dose should be doubled to 50 mg/day (spironolactone) or 16 mg/day (doxazosin) with a more concentrated beetroot juice (9 mmol nitrate/day or control, 0 mmol nitrate/day), depending on biochemical and BP data at check up visits. Visits 6 and 7 (V6, V7 at 24 and 36 weeks respectively), follow the same assessments as at V2 and V5; at one of these visits the study is terminated, depending on the length agreed with the participant at the start of the study, with study duration being shorter as recruitment nears its end (Fig. 1).
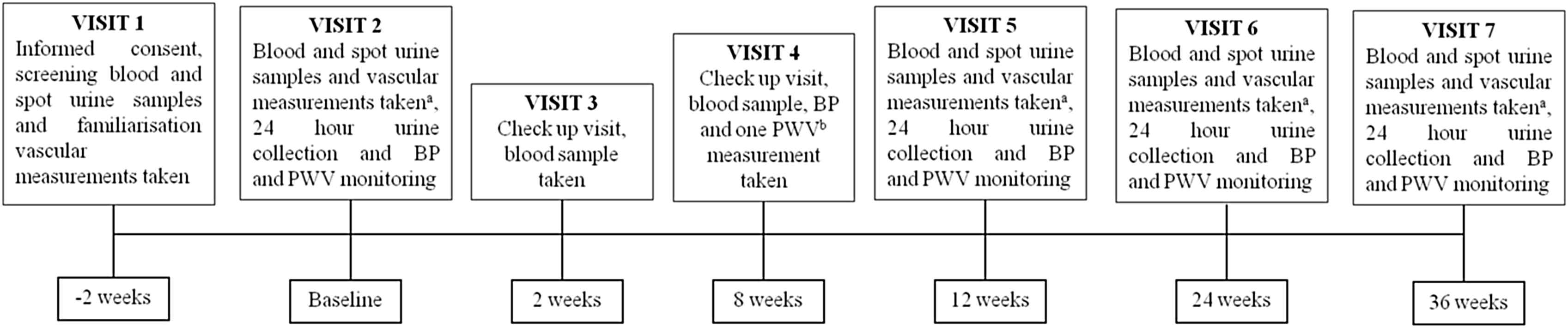
Study flow diagram. aMultiple arterial stiffness measurements; central (ao) pulse wave velocity (PWV) by Arteriograph, carotid femoral (cf) PWV by Vicorder and cardio-ankle vascular index (CAVI) by VaSera, in addition to echocardiogram and blood pressure monitoring. bOne off aoPWV by Arteriograph. PWV; pulse wave velocity, BP; blood pressure.
The study was reviewed and approved by Central London National Research Ethics Service (NRES).
Participant eligibility
We aim to randomise up to 120 people with or at risk of T2D from the local community (South London, UK) with a broad inclusion criteria for eligibility. Recruitment is carried out through referral from hypertension and diabetes clinics in secondary care, appropriate contact with local health centres and general practices and advertisements. The aim is to recruit approximately equal numbers of men and women and equal numbers with T2D and those at risk of T2D. The following inclusion and exclusion criteria are followed on recruitment of participants.
Inclusion criteria:
- -
Age 18–80 years
- -
Previously clinically diagnosed T2D or at risk of T2D (body mass index (BMI) ≥ 27 kg m−2, a positive family history or glucose intolerance 2 h after (75 g challenge)
- -
Ability to understand the protocol and willingness to comply with it
Exclusion criteria:
- -
Chronic illness of any type that may interfere with participation
- -
Previous adverse reaction to spironolactone or doxazosin
- -
Known allergy to beetroot
- -
Impaired renal function (eGFR < 45 mL min−1)
- -
HbA1c > 11% or fasting random glucose > 12 mmol L−1
- -
Being pregnant or breastfeeding for the duration of the study
- -
Atrial fibrillation
Arterial stiffness techniques
AS is measured by a variety of devices, which have demonstrated good repeatability (between V1 and V2) at V1, V2, V5, V6, and V7 to assess the efficacy of the interventions on AS. Participants are measured using:
- i)
The VaSera VS-1500N (Fukuda Denshi Co., Ltd, Japan), a four-cuff device (placed over the upper arms, as with a standard BP cuff, and above both ankles) measuring stiffness from the heart to the ankles, using a microphone to detect aortic valve closure (the 2nd heart sound) expressed as CAVI a value calculated from PWV using the stiffness parameter, β. The CAVI formula (Fig. 2), which greatly reduces the dependence of PWV on ambient BP, aims to give a BP independent estimate of arterial stiffness. A change in CAVI is the primary outcome of the study.
- ii)
The Arteriograph 24™ (Tensiomed Kft. Hungary), which measures aoPWV using a non-invasive, one cuff method. The device uses the return time (difference between the first and reflected systolic peaks) and distance between sternal notch and symphysis to calculate aoPWV. The device has been calibrated against cfPWV and MR derived PWV with length calibration.19
- iii)
The Vicorder® (SMT medical GmbH & Co. KG, Germany) is another non-invasive cuff based technique to measure carotid-femoral cfPWV; one cuff, a small band on the maximal carotid pulsation on one side of the neck only to detect carotid pulse waves (and easily striped off if the participant feels claustrophobic), the other around the upper thigh to detect femoral pulse waves.
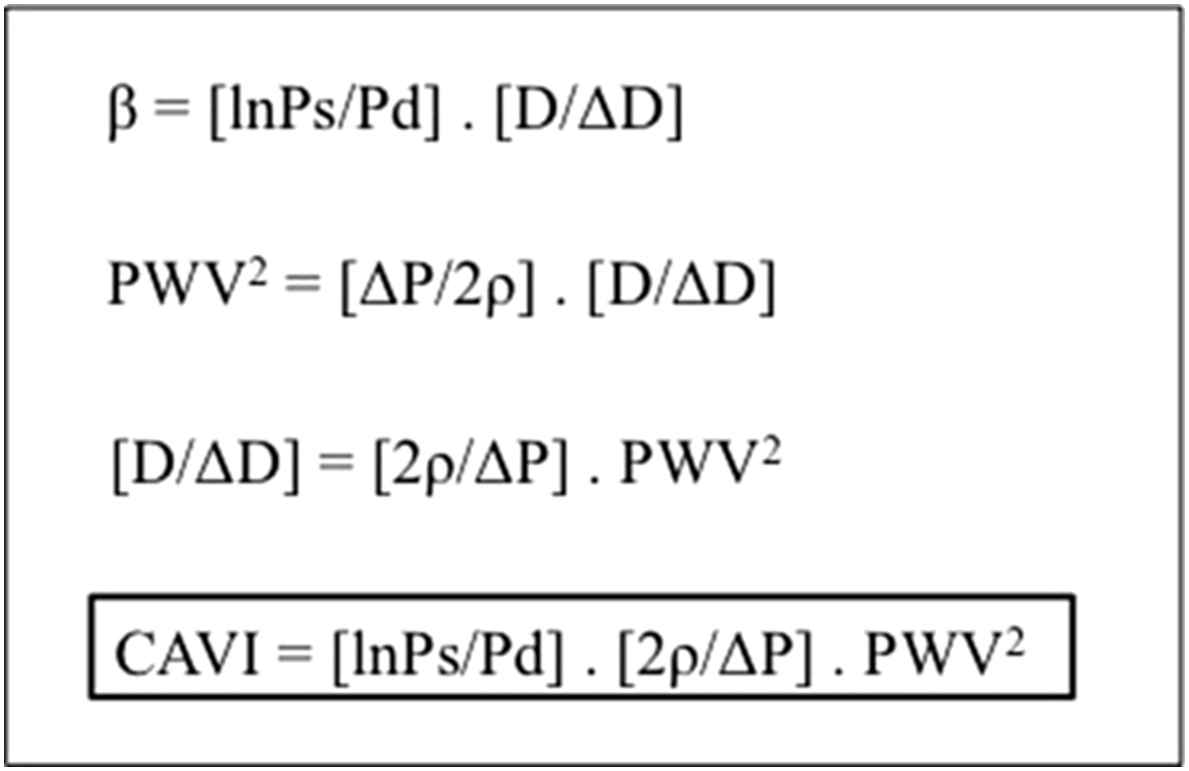
Formulae to derive cardio-ankle vascular index (CAVI). β; stiffness parameter, Ps; systolic pressure, Pd; diastolic pressure, D; diameter, PWV; pulse wave velocity, P; pulse pressure, ρ; blood density, CAVI; cardio-ankle vascular index.
Hypothesis
We hypothesise that between spironolactone and doxazosin (which both lower BP), spironolactone will further alter CAVI by ≥0.4 units and CAVI’s cardiac-brachial PWV and aoPWV by ≥0.5 ms−1 with a minimal difference in BP. Further, we anticipate that the oral inorganic nitrate supplement as the beetroot juice will promote longer term generation of vascular nitric oxide (measurable as increased circulating nitrite) and also, independently of the drug comparison arm, reduce PWV by ≥0.5 ms−1, again after adjusting for any change in BP, compared with the effect of the control juice with no nitrate. We hypothesise that there will be an additive effect ≥1 ms−1 of both the spironolactone and nitrate in the combined arm, compared with neither on the assessed vascular parameters.
Statistical considerations & power
Sufficient data were not available from which to calculate sample sizes directly for CAVI only. Therefore to detect a reduction in CAVI, of 20% (±8% standard deviation) with minimum 80% power, at p < 0.05 over 6 months of on the study, we based calculations on previous short term studies on BP with beetroot juice12,14 and a long term study on aoPWV and spironolactone,8 at similar power, and estimated that 24 participants per box of the 2 × 2 table are required. We therefore aim to recruit 30 patients per group based on a 20% dropout rate, to generate 25 people per box to finish the trial.
Results
Participant descriptives
On writing this manuscript, 95 patients had been consented, of which 7 were unsuitable due to uncontrolled diabetes, adverse reactions to the study interventions and being unable to commit. A dropout rate of 14% between consent and randomisation and 7% after randomisation has been observed, see CONSORT diagram, Fig. 3. To that date, 53 participants have completed the entire study. The baseline descriptives of participants who entered to date at randomisation in Table 2 (n = 73) show that we have recruited slightly more men than women, and more with clinically diagnosed T2D than those at risk. We classified ethnicity by local populations known to be at higher risk of diabetes (West African and African Caribbean, with relatively few South Asian people living locally).
| Parameter | Men ± SD | Women ± SD |
|---|---|---|
| n (%) | 62 | 38 |
| Age (years) | 58 ± 13 | 61 ± 11 |
| Weight (kg) | 96.3 ± 19 | 87.5 ± 19.3 |
| BMI (kg m−2) | 32 ± 6 | 34 ± 7 |
| Waist circumference (cm) | 109 ± 12 | 107 ± 16 |
| Type 2 diabetes (%) | 73 | 75 |
| Ethnicity (%): European | 60 | 54 |
| African Caribbean | 4 | 18 |
| West African | 4 | 18 |
| Other | 31 | 11 |
| SBP (mm Hg) | 133 ± 17 | 129 ± 16 |
| DBP (mm Hg) | 78 ± 10 | 74 ± 10 |
| Pulse (BPM) | 70 ± 12 | 69 ± 12 |
| aoSBP (mm Hg) | 128 ± 22 | 125 ± 18 |
| aoAIX (%) | 25 ± 17 | 31 ± 14 |
| aoPWV (m s−1) | 9.21 ± 1.81 | 9.31 ± 1.67 |
| cfPWV (m s−1)a | 8.90 ± 1.60 | 8.78 ± 1.37 |
| CAVI (units) | 8.40 ± 1.45 | 7.81 ± 0.95 |
BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; BPM, beats per minute; ao, aortic (central); cf, carotid-femoral; ca, cardio-ankle; CAVI, cardio-ankle vascular index.
Men, n = 27 and women, n = 21 for this measurement.
Baseline characteristics of 52 participants who have completed the study to date.
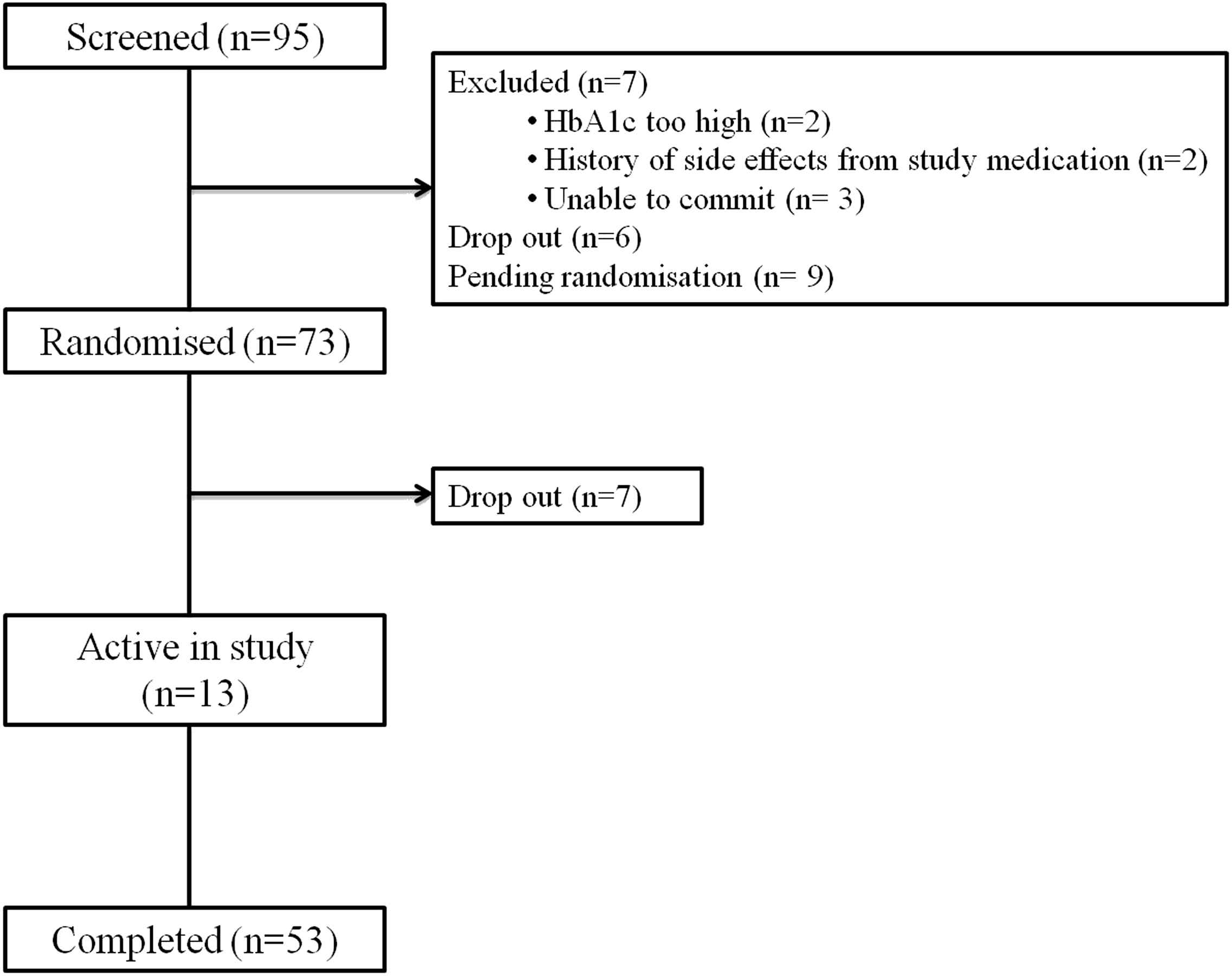
CONSORT (consolidated standards of reporting trials) diagram. Based on inclusion/exclusion criteria patients are diagnosed with or at risk of type 2 diabetes (with BMI ≥27 kg m−2 or glucose intolerance). HbA1c are to be <11% or fasting glucose <12 mmol L−1 with good renal function (eGFR >45 mL min−1).
Considering the associations with vascular health, despite recruiting participants with or at risk of T2D the mean blood pressure is not elevated; our participants are not clinically defined as hypertensive. However, the mean PWV and CAVI of the participants, as measured by all methods suggest there is some stiffening of the arteries, for the men assessed this is even above the reference value considering their age.21
Study interventions
Of the 73 participants who have been randomised onto the study, there have been 103 reported adverse events (from 57 participants) post randomisation. Of these events 23 (from 12 participants) are considered to be attributed to the interventions, with common reported events being dizziness/lethargy (13% of randomised participants), nausea/sickness or diarrhoea (4%), incontinence (4%), rash (3%). No participants have dropped out of the study due to reported adverse effects from the interventions; all effects were controlled by reduction in dose or by stopping one of the interventions. In addition to these there have been six reported serious adverse events post randomisation with none of these being attributed to the interventions.
Conclusions
This study was designed to investigate the effect of inorganic nitrate from beetroot juice and spironolactone on arterial stiffness, independent of blood pressure in those with or at risk of T2D. To date this study has proven easy to recruit for with a low dropout rate (7% after randomisation). Participants have been happy taking the interventions with minimal consequential ill effects, and have generally been committed to the study for the duration.
Funding
This work was funded by an unrestricted grant from
Conflict of interest
JKC and AW report an unrestricted grant from Fukuda Denshi co. Ltd to support this work and JKC fees for lecturing. AW also reports a small share holding in Heartbeet Ltd.
All other authors certify that they have no affiliations with or involvement in any organisation or entity with financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
References
Cite this article
TY - JOUR AU - Charlotte E. Mills AU - Virginia Govoni AU - Maria Linda Casagrande AU - Luca Faconti AU - Andrew J. Webb AU - J. Kennedy Cruickshank PY - 2015 DA - 2015/11/06 TI - Design and progress of a factorial trial testing the effect of spironolactone and inorganic nitrate on arterial function in people at risk of or with type 2 diabetes JO - Artery Research SP - 48 EP - 53 VL - 12 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2015.10.194 DO - 10.1016/j.artres.2015.10.194 ID - Mills2015 ER -
