Frequency of multi-drug resistance and mutations in Mycobacterium tuberculosis isolates from Punjab state of India
- DOI
- 10.1016/j.jegh.2017.05.002How to use a DOI?
- Keywords
- MDR suspect; Genotype MTBDRplus; Mutation
- Abstract
Data regarding prevalence of multi-drug resistant tuberculosis (MDR-TB) and associated common mutations is scarce from Punjab region. The study was designed to determine rate of MDR-TB among presumptive MDR-TB from Punjab and mutation patterns using GenoType MTBDRplus assay. Total of 812 consecutive sputum samples were received from January 2012 to July 2013, from 14 districts of Punjab at the National Reference Laboratory at New Delhi for diagnosis of MDR-TB as hand holding activity. Presumptive MDR-TB patients were identified on basis of criterion B defined by the programme. Smear positive and negatives patients were found to be 636/798 (79.7%) and 162/ 798 (20.3%) respectively. Total of 606 GenoType MTBDRplus tests were conducted and mutations in rpoB, kat G and inhA genes analyzed. Total of 94/606 (15.5%), 43/606 (7.1%) and 40/606 (6.6%) were found to be RIF and INH resistant, mono-RIF resistant and 40/606 (6.6%) mono-INH resistant respectively. Commonest known mutation for RIF in rpoB gene and INH in kat G gene was S531L (80/ 137; 58.4%) and S315T1 (119/134; 88.8%) respectively. Mutations in inhA were found in 21/134 (15.7%) strains. Average turn-around time (TAT) for dispatch of result toPunjab was 4.6 days. Prevalence of RIF resistance in Punjab was found to be 22.6%. Common mutations for RIF and INH were similar to that in other regions of country. GenoType MTBDRplus was found to be useful assay for rapid detection of MDR-TB, responsible for determining better management of MDR-TB patients under the programme.
- Copyright
- © 2017 Ministry of Health, Saudi Arabia. Published by Elsevier Ltd.
- Open Access
- This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Multi-drug resistant tuberculosis (MDR-TB), defined as the resistance to at-least rifampicin (RIF) and isoniazid (INH), continues to be threatening as worldwide, 3.9% of new TB cases and 21% of previously treated cases are estimated to have MDR-TB. In 2015, 580, 000 cases were eligible for MDR-TB treatment. India, China and Russian Federation accounted for 45% of the cases [1]. Almost 10% to 30% of MDR-TB cases result in failure of treatment and death due to prolonged, limited and expensive treatment options.
Considering the numbers described above, it is imperative to perform drug susceptibility testing (DST) for appropriate management of drug resistant cases. The culture based DST methods are based on inhibition of organisms in culture medium containing anti-tubercular agents and take weeks or months to be completed. While culture based DST is ongoing, the undiagnosed drug resistant cases continue to spread resistance [2].
Many molecular techniques for determination of anti-tubercular resistance based on genetic mutation have been introduced in the last decade. Commercial line probe assays (LPA) such as INNO-LIPA Rif TB (Innogenetics, Ghent, Belgium) and Genotype MTBDR plus assay (Hain Life Sciences, Nehren, Germany) based on reverse hybridization of amplicons to immobilized membrane based probes are designed to simultaneously detect M. tuberculosis (MTB) isolates and important gene mutations conferring rifampicin (RIF) resistance (rpoB gene) and isoniazid (INH) resistance (inhA, katG) within 2–3 days [2,3].
Genotype MTBDR plus can be used for detection of resistance directly on the smear positive sputum samples or the culture isolate and has pooled sensitivity and specificity of 98.1% and 98.7% for RIF resistance and 84.3% and 99.5% for INH resistance respectively [4]. The RIF resistance is caused by altered beta-subunit of DNA dependent RNA polymerase, caused by mutations commonly found in 81-bp hot-spot region of rpoB gene. Resistance to INH is most frequently associated with a specific mutation S315T in katG gene coding for catalase-peroxidase and/ or C15T, A16G, T8A & T8C in the promoter region of inhA gene coding for nicotinamide-adenine dinucleotide phosphate-oxidase enoyl-acyl carrier protein reductase [5]. These mutations are visible in form of absence of wild type regions and/or presence of specific mutations which in addition provide the knowledge of mutations.
Another path-breaking fully automated molecular assay is Cepheid GeneXpert® MTB/RIF, in which real time polymerase chain reaction technology is used to simultaneously detect MTB and RIF resistant mutation in rpoB gene. Xpert requires minimal laboratory expertise, results are available in less than 2 h and sensitivity of detection of MTB is about 130 organisms/ ml [6]. Cepheid is now launching Xpert MTB/RIF Ultra, in which limit of detection is 10–100 organisms/ml. India targets rapid detection of MDR-TB for all possible suspects using LPA as envisioned under Programmatic Management of Drug Resistant Tuberculosis (PMDT), across the country [7]. The patients are referred to the designated Reference Laboratory for diagnosis of MDR-TB. Punjab state is a north-western state of India with population of 27.98 million with 22 districts. Since past few years, Punjab has witnessed very high substance abuse, which has also been epidemiologically linked to the disease [8].
Few studies have reported prevalence of M. tuberculosis genotypes and MDR-TB rates using LPA from North India [9,10]. Therefore, present study targets to study drug resistance TB and the frequency of various mutations detected among these cases using LPA in Punjab.
2. Materials and methods
2.2. Study setting
The present study is conducted in the National Reference Laboratory (NRL) for TB, Department of Microbiology at National Institute of Tuberculosis and Respiratory Diseases (NITRD), New Delhi. Laboratory is certified as NRL for M. tuberculosis DST by Supranational Laboratory (Antewerp Belgium) and recognized as Centre of Excellence by World Health organization.
2.3. Study design
As hand holding activity, samples from 14 different districts of Punjab; Amritsar, Fatehgarh Sahib, Firozpur, Gurudaspur, Hoshiarpur, Jalandhar, Ludhiana, Moga, Mohali, NawanShahar, Patiala, Rupnagar, Sangrur and SAS Nagar were received at the NRL for diagnosis of MDR-TB till state developed complete capacity for the same (Fig. 1). Total of 812 consecutive sputum samples from patients identified as presumptive MDR-TB, received in cold chain from January 2012 to July 2013, were included in the study. The presumptive MDR-TB patients were identified on basis of criterion B of PMDT which include follow up positives on category I and II, smear positive retreatment cases and contacts of MDR-TB. The study is approved by institute’s ethical committee (AMS/EC/2012/1028 dated: 5/2012).
Different districts of Punjab state in North-West India.
2.4. Sample processing
All specimens were screened for presence of acid fast bacilli (AFB) by Ziehl-Neelsen (ZN) staining [11]. Samples were processed by N-acetyl-L cysteine – Sodium hydroxide (NALC-NaOH) method of digestion and decontamination [11]. All smear negative processed samples and scanty smear positive (Only when Genotype MTBDRplus assay ver 1.0 was used) were inoculated in MGIT 960 tubes for culture as per PMDT guidelines [7]. Tubes with positive alerts were identified for presence of M. tuberculosis by smear microscopy for serpentine cording and rapid immuno-chromatographic test for detection of MPT64 TB Ag (SD BIOLINE). Cultures positive for M. tuberculosis were subjected to LPA. The study workflow is shown in Fig. 2.
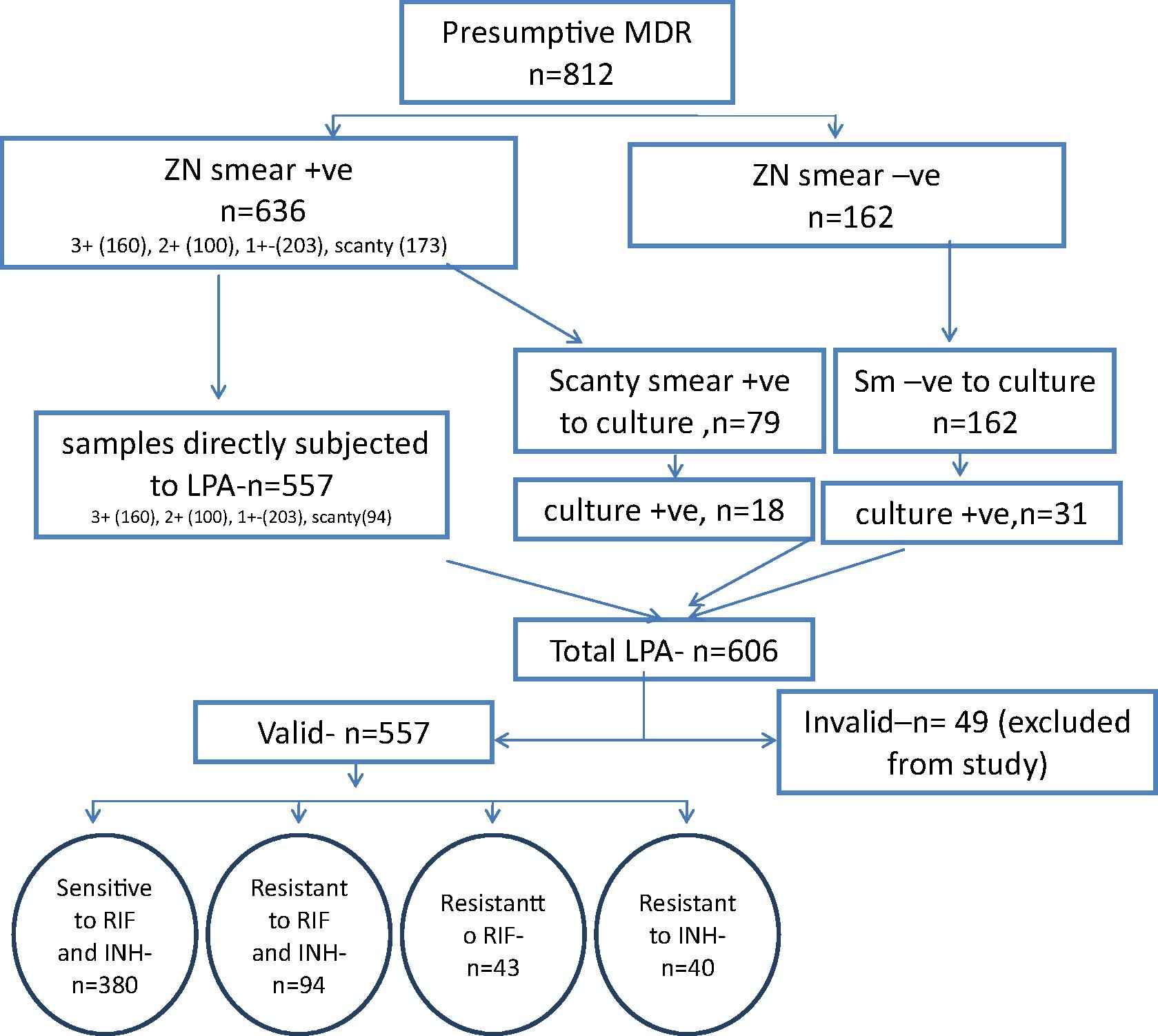
Process flow of the study.
2.5. GenoType MTBDRplus assay/line probe assay
Smear positive processed sputum samples were directly processed for GenoType MTBDRplus assay [7]. The Genotype MTBDRplus assay ver 1.0 or ver 2.0 (Hain Life Sciences, Nehran, Germany, was carried out on the processed sample as per manufacturer’s instructions (http://www.hain-lifescience.de). DNA extraction, master-mix preparation, DNA amplification and hybridization were done after thorough cleaning in dedicated rooms [10,12]. The M. tuberculosis H37Rv (ATCC 27294) was run as positive control and sterile molecular grade water was run as negative control, for quality control.
For DNA extraction, 500 μl of NaLC-NaOH processed samples or 1 ml of M. tuberculosis cultures from MGIT tubes was taken in cryo-vial, centrifuged at 10,000g for 15 min and bacterial pellets obtained. In version 1.0, pellets were suspended in 100 µl sterile molecular grade water, heat inactivated at 95 °C for 20 min followed by ultra-sonication for 15 min. Supernatant containing DNA was collected after centrifugation at 13,000g for 5 min. In version 2.0, Genolyse kit was used and 100 µl of lysis buffer was added to pellet, heat inactivated at 95 °C for 5 min followed by addition of neutralization buffer. Supernatant containing DNA was collected after centrifugation at 13,000g for 5 min in fresh tube and stored at − 20 °C (http://www.hain-lifescience.de).
Master-mix was prepared using reagents provided by the kit. In version 1.0, master-mix was prepared by mixing 35 µl polynucleotide mix, 2 µl MgCl2 buffer, 5 µl buffer, 3 µl water, and 0.2 µl taq polymerase. In version 2, the above constituents have been condensed as AM-A & AM-B which are mixed for each sample in ratio of 10 µl and 35 µl respectively. DNA solution is added in volume of 5 μl to PCR tubes and amplified.
Detection of PCR products was done using LPA based hybridization using GenoType MTBDRplus kit. Initially denaturation of amplified DNA was done, followed by hybridization of nucleotides on single stranded DNA strand to LPA strip, followed by conjugate reaction and finally addition of substrate to give the bands on LPA strip (http://www.hain-lifescience.de).
2.6. Reporting
The result of LPA strips was interpreted with the help of reporting card as resistant or sensitive for RIF or INH or invalid based on the kit insert. Resistance to particular antibiotic was considered if one or more wild type (WT) bands were missing or/and one or more mutant bands (MUT) were present. Strains with presence of both wild type and mutant band were termed as hetero-resistance.
The reports were communicated electronically to the District TB Officers (DTO) of the respective districts within 24 h of report generation. Turn-around time (TAT) for each sample was calculated.
2.7. Statistical analysis
Data was presented as frequency tables and mean was calculated wherever required using MS-Excel. Fisher’s exact test for statistical significance (Defined as the probability that an effect is not due to by chance alone) was calculated wherever applicable using Graph-pad computer software (https://www.graphpad.com/quickcalcs/contingency1.cfm). The P value less than 0.05 was considered significant.
3. Results
Total of 812 samples were received from various districts of Punjab from January 2012 to July 2013. Male to female ratio was 2.7:1 as 596 subjects in the study were males. The pediatric and geriatric patients were 30 and 77 respectively. Most patients were found in the age groups of 16–35; 360 (44.3%) and 36–60; 345 (42.5%).Median age was 37 years (6–80). Fourteen samples were found to be inadequate on account of leakage or insufficient quantity and were not processed further.
Smear positive and smear negatives patients were found to be 636/798 (79.7%) and 162/ 798 (20.3%) respectively. The distribution of smear result is detailed in Table 1. Total of 606 GenoType MTBDRplus tests were conducted. Of these, 557 were done directly on samples and 49 on positive cultures. Valid LPA results were obtained for 557/ 606 (91.9%) LPA performed. 94/557 (16.9%) tests were found to be resistant to RIF and INH, 43/557 (7.7%) as mono-RIF resistant, 40/557 (7.1%) as mono-INH resistant and 380/557 (68.2%) as sensitive to both RIF and INH (Table 1).
| Smear status | Sample received | Total LPA done | LPA conducted (Sample) | LPA conducted (Culture) | Valid (%) | MDRa (%) | RIFb Resistant (%) | INHc Resistant (%) | Both Sensitive (%) |
|---|---|---|---|---|---|---|---|---|---|
| 3+ | 160 | 160 | 160 | 0 | 158(98.8%) | 33 (20.9%) | 15 (9.5%) | 13 (8.2%) | 97 (61.4%) |
| 2+ | 100 | 100 | 100 | 0 | 98 (98%) | 20 (20.4%) | 8 (8.2%) | 7 (7.1%) | 63 (64.3%) |
| 1+ | 203 | 203 | 203 | 0 | 181 (89.1%) | 25 (13.8%) | 14 (7.7%) | 15 (8.3%) | 127 (70.2%) |
| Scanty | 173d | 112 | 94 | – | 71 (75.5%) | 5 (7.0%) | 1 (1.4%) | 4 (5.6%) | 61 (85.9%) |
| – | 18 | 18 (100%) | 8 (44.4%) | 2 (11.1%) | 0 | 8 (44.4%) | |||
| 89 (79.5%) | 13 (14.6%) | 3 (3.4%) | 4 (4.5%) | 69 (77.5%) | |||||
| Negative | 162e | 31 | – | 31 | 31(100%) | 3 (9.7%) | 3 (9.7%) | 1(3.2%) | 24 (77.4%) |
| Total | 798 | 606 | 557 | 49 | 557(91.9%) | 94 (16.9%) | 43 (7.7%) | 40 (7.1%) | 380 (68.2%) |
MDR: Multi-drug resistant.
RIF: Rifampicin.
INH: Isoniazid.
Out of 79 samples put for culture, 6 were contaminated, 55 culture negative, 18 culture positive.
Out of 162 samples put for culture, 13 were contaminated, 118 culture negative, 31 culture positive.
Distribution of multi-drug resistant, mono-rifampicin resistant, mono-isoniazid resistant and sensitive strains as per smear status.
The MDR-TB/ RIF resistance strains were three times commoner in males as compared to females. The MDR-TB/ RIF resistance rate detected among higher smear grade (2 + and 3+); 76/ 256; 29.7% was found to be significantly higher than among lesser smear grades of 1+, scanty positive and negative 61/ 301; 20.3% (P value = 0.01). For LPA performed on samples, the invalid rate decreased with increase in AFB concentration 2/160 (1.2%); 3+, 2/ 100 (2.0%); 2+, 22/203 (10.1%); 1+ and 23/94 (24.5%); scanty. This correlation was statistically highly significant (P value < 0.00001). Overall, 49/ 557 (8.8%) of tests performed on samples were invalid. Average TAT was found to be 4.6 days and 29.6 days for LPA performed on the sample and from culture respectively.
Among 137 RIF resistant strains, known mutations in rpoB were found in 100 (73%) strains. (Table 2). Commonest known mutation was S531L in (80/ 137; 58.4%) followed by D516V (8/ 137; 5.8%), H526Y (7/ 137; 5.1%) and H526D (5/137; 3.6%). In 37/137 (27%) of RIF resistant strains, wild type probes were missing without known mutations. These included missing wild type bands WT2 (11/ 37; 29.7%), W3-W4 (11/ 37; 29.7%), WT8 (6/37; 16.2%), WT7 (3/37; 8.1%), WT4 (2/120; 5.4%), WT1 (1/37; 2.7%), WT1-WT5-WT6 (1/37; 2.7%) (Table 2). Hetero-resistance to RIF with presence of wild type along with mutant probes was found in (5/137; 3.6%) cases.
| S. No | Band Missing (WT a)/Mutation present (MUT b) | Gene Region/specific mutation rpoB | MDR e (n = 94) | Mono RIF f resistant (n = 43) | Total RIF resistant (n = 137) | Mono INH g resistant (n = 40) | Total INH resistant (n = 134) | P value |
|---|---|---|---|---|---|---|---|---|
| 1 | rpoB | |||||||
| 2 | WT1 | 506–509 | 0 | 1 | 1 | – | ||
| 3 | WT2 | 510–513 | 7 | 4 | 11 | – | ||
| 4 | WT4 | 517–519 | 2 | 0 | 2 | – | ||
| 5 | WT1,WT4 | 506–509,517–519 | 2 | 2 | ||||
| 6 | WT3,WT4 | 513–517,517–519 | 9 | 2 | 11 | – | ||
| 7 | WT1, WT5,WT6 | 506–509, 518–522,521–525 | 0 | 1 | 1 | |||
| 8 | WT7 | 526–529 | 3 | 0 | 3 | – | ||
| 9 | WT8 | 530–533 | 5 | 1 | 6 | – | ||
| 10 | UKc | 28 | 9 | 37 | 0.3 | |||
| 11 | MUT 1 | D516 V | 6 | 2 | 8 | 1 | ||
| 12 | MUT2A | H526Y | 5 | 2 | 7 | 1 | ||
| 13 | MUT2B | H526D | 4 | 1 | 5 | 1 | ||
| 14 | MUT3 | S531 L | 51 | 29 | 80 | 0.2 | ||
| 15 | Total | 94 | 43 | 137 | ||||
| 16 | katG | |||||||
| 17 | WT | 315 | 5 | 3 | 8 | – | ||
| 18 | UKc | 5 | 3 | 8 | – | |||
| 19 | MUT1 | S315T1 | 81 | 29 | 110 | 0.08 | ||
| 20 | MUT2 | S315T2 | 1 | 0 | 1 | – | ||
| 21 | Total | 87 | 32 | 119 | 0.07 | |||
| 22 | inhA | |||||||
| 23 | WT1 | 15/16 | 1 | 0 | 1 | – | ||
| 24 | WT2 | 8 | 0 | 0 | 0 | – | ||
| 25 | UKc | 1 | 0 | 1 | – | |||
| 26 | MUT1 | C15T | 8 | 9 | 17 | 0.04 | ||
| 27 | MUT2 | A16 G | 0 | 0 | 0 | – | ||
| 28 | MUT3A | T8C | 2 | 1 | 3 | – | ||
| 29 | MUT3B | T8A | 0 | 0 | 0 | – | ||
| 30 | Total | 11 | 10 | 21 | 0.06 | |||
(−) p value not calculated due to insignificant numbers.
S. No 2–10, 17,18, 23–25 denote mutations due to missing wild type without specific mutation.
S. No 11–14, 19, 20, 26–29 denote specific mutations.
S. No 15, 21 and 30 denote total mutations in rpoB, katG and inhA genes respectively.
MDR: Multi-drug resistant; resistant to both rifampicin & isoniazid.
WT: Wild type.
MUT: Mutant.
UK: No known mutations as defined by the kit.
RIF: Rifampicin.
INH: Isoniazid.
Mutations detected by GenoType MTBDRplus assay in rifampicin or isoniazid resistant Mycobacterium tuberculosis.
The katG mutation occurred in 119 (88.8%) of 134 INH resistant strains detected, of which known mutation S315T1 was found in 110/119 (92.4%) of katG mutant strains. Missing wild type without specific mutation in katG was found in 8/119 (6.7%) cases. The inhA mutations were found in 21/134 (15.7%) of INH resistant strains which included C15T (17/21; 81.0%), T8C (3/21; 14.3%). Missing wild type with no mutation band were seen in 1/21; 4.7% cases. Mutations in C15T of inhA were significantly higher in INH mono-resistant than MDR strains (P value < 0.04). Both inhA and katG mutations were seen in 7/134 (5.2%) of isolates (Table 2). Hetero-resistance pattern to INH was found in 6/134 (4.5%) cases, 4 in katG & 2 in inhA.
4. Discussion
Timely and accurate diagnosis of MDR-TB is imperative towards control of the disease. Drug resistance determination based on WHO endorsed newer genotypic methods such as LPA and Gene Xpert has revolutionized TB and DR-TB diagnostics over last decade by bringing down laboratory TAT to 1–3 days [12,13]. The reduced TAT has attributed to major decrease in the delay between identification of patients suspected for MDR-TB and initiation of treatment [14]. The methods also give detailed and precise information about mutations responsible for resistance which are found to correlate with the sequencing results as well as standard phenotypic DST [15,16]
The present manuscript highlights the resistance rate of RIF and INH using LPA and genetic mutations in rpoB, katG and inhA genes in the samples received from Punjab in North Indian RNTCP network.
As also reported by Sharma et al. earlier, maximum patients were found in young age group of 16–35; 360 (44.3%) and males were predominant with 74.4% [17]. The MDR-TB in this age-sex group translates into significant socio-economic implications as young adult males form the economically productive strata of population.
High MDR-TB rate was obtained (16.9%), similar to 16.6% as seen in recent study from Delhi region when in criteria B [10]. Another study from region reported even higher rate of MDR-TB (24.1%) [9]. High rate of MDR-TB reported could be due to highly suspected target population and due to rampant use of substance use drugs in the state [8].
World-wide surveillance of MDR-TB in re-treatment cases is reported as 9.4% to 36.5% [18]. In India, MDR-TB rates in retreatment cases is from 17.4% to 53% [19,20]. Exposure to anti-tuberculosis agents promotes development of mutations and drug resistance in MTB.
The MDR-TB/ RIF resistance rates decreased significantly with bacillary load with 29.7% in higher smear grade (2+ and 3+) samples and 20.3% in lower smears. Correlation of MDR-TB with bacillary load needs to be substantiated with more studies.
The reported invalid results were within the acceptable limits and decreased significantly as the bacillary load increased from scanty to 3+. This has been reported earlier as well [10,12]. The sensitivity of the assay for MTB detection is highly dependent on bacillary load and hence has been recommended for smear positive samples. Genotype MTBDRplus ver 2.0 kit has better sensitivity than ver 1.0 and gives valid results in most scanty positive patients. Also, in ver 2.0, bands in inhA gene are better defined.
The known mutations were identified in 73% of RIF resistance isolates, similar to the study from New Delhi [10], though significantly different from an Indian study; 55.2% and with a South African study; 91% [21,12]. Mutation S531L was detected in 58.4% of RIF resistance cases, similar to other Indian studies; 59.8%, 59% [10,22]. However, internationally wide variations ranging from 47% to 70.5% have been found [3,12,23]. None of the mutations in rpoB gene were found to be significantly different among MDR-TB or mono-RIF resistant strains [10,12]. Information about the mutations causing resistance is covered well by GenoType MTBDRplus and correlates highly with the sequencing results [15].
The missing wild type probe without any mutant bands was found in 27% of RIF resistant isolates, similar to other Indian and International studies, New Delhi; 26.1%, France; 29%, Vietnam; 33.3%. This could be because, GenoType MTBDRplus incorporates the probes for common mutations only [10,3,23].
The katG gene mutations, responsible for high-level INH resistance account for commonest mechanism of resistance for INH. Specific mutations in katG, were found in high proportion of almost 93.2% of INH resistant isolates, similar to most studies, however deviations are reported in studies from France; 62.5% and South Africa; 37.6% [3,12]. These katG mutations contribute to most cases of INH resistance in high burden countries. There was no significant difference in prevalence of katG mutations in MDR strains as compared to Mono-INH resistant strains which was found to be higher in Delhi [10].
Mutations in inhA gene, responsible for low-level INH resistance were found to be in 15.7% cases, similar to many studies elsewhere 5.4% to 21.1% [3,10,23,24]. Current assay offers additional detection of INH resistant strains of about 15–20% due to mutations in inhA gene, which was lacking in the earlier version; Genotype MTBDR assay.
Hetero-resistance, i.e. presence of all wild type probes along with presence of one or more mutant bands was found in 3.6% of RIF resistant strains and 3% of INH resistant strains. This rate is in accordance with most studies [10]. Another study, from Punjab, has reported high number of hetero-resistant strains of 28.8% for RIF in rpoB and 9.8% for INH in katG [9]. Timely detection of hetero-resistant strains ensures timely initiation of MDR-TB regimens in such patients. Patients harboring such strains would have better treatment outcomes compared to pure MDR-TB patients [24].
5. Conclusion
In conclusion, high prevalence of 24.6% for RIF resistance in Punjab was found. Hence it appears necessary to perform more studies on drug resistance detection, specifically directed towards the population with substance use. Furthermore, GenoType MTBDRplus has revolutionized MDR-TB diagnosis by reducing TAT and providing high throughput system, which promotes prompt detection and management of drug resistant cases. Additional information about common mutations imparting resistance to RIF and INH, helps in understanding the disease epidemiology in various regions.
Contribution of each author
Dr. Ritu Singhal: Study Design, written the manuscript, data analysis & interpretation.
Dr. Jyoti Arora: Data analysis & interpretation.
Mr. Grish C. Sah: Data compiling, performing the LPA test.
Dr. Manpreet Bhalla: Editing the manuscript.
Dr. Rohit Sarin:Editing the manuscript.
Dr. Vithal Prasad Myneedu: Providing logistics support, concept.
References
Cite this article
TY - JOUR AU - Ritu Singhal AU - Jyoti Arora AU - Grish C. Sah AU - Manpreet Bhalla AU - Rohit Sarin AU - Vithal Prasad Myneedu PY - 2017 DA - 2017/05/24 TI - Frequency of multi-drug resistance and mutations in Mycobacterium tuberculosis isolates from Punjab state of India JO - Journal of Epidemiology and Global Health SP - 175 EP - 180 VL - 7 IS - 3 SN - 2210-6014 UR - https://doi.org/10.1016/j.jegh.2017.05.002 DO - 10.1016/j.jegh.2017.05.002 ID - Singhal2017 ER -
