Aerobic exercise training increases cerebral blood flow in postmenopausal women
- DOI
- 10.1016/j.artres.2012.05.003How to use a DOI?
- Keywords
- Physical activity; Cerebral hemodynamic; Aging
- Abstract
Background: Age-related decrease in cerebral blood flow is a risk factor for cognitive decline. Aerobic exercise training improves vascular and cognitive functions. However, the effect of exercise training on age-related reduction in cerebral blood flow is unclear. The present study investigated whether aerobic exercise training can increase cerebral blood flow in postmenopausal women.
Methods: Twenty healthy postmenopausal women were assigned to either the exercise training group (n = 10) or the control group (n = 10). The exercise group completed 8 weeks of moderate aerobic exercise intervention. The control group did not change their physical activity level. Before and after each intervention, middle cerebral blood flow velocity and cerebrovascular resistance were measured using transcranial Doppler ultrasonography.
Results: The baseline middle cerebral blood flow velocity, cerebrovascular resistance, and most other key dependent variables did not significantly differ between the groups. Aerobic exercise training significantly increased middle cerebral blood flow velocity and significantly decreased cerebrovascular resistance, whereas no such changes were observed in the control group.
Conclusions: We showed that aerobic exercise training increased middle cerebral blood flow velocity and decreased cerebrovascular resistance in postmenopausal women. The results of the current study suggest that regular aerobic exercise may attenuate aging-induced decrease in cerebral blood flow.
- Copyright
- © 2012 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Cerebral blood flow decreases with aging.1,2 Cerebral hypoperfusion decreases oxygen delivery and metabolic function in vulnerable brain areas such as the hippocampus, which results in neurodegeneration, apoptosis, and cognitive decline.3,4 Several studies have shown that patients with dementia have decreased cerebral blood flow5–7 and that low cerebral blood flow is a risk factor for cognitive decline in subjects without dementia.8 As cerebral hemodynamics are partly modulated by sex hormones, postmenopausal depletion of estrogens is believed to contribute to cerebrovascular dysfunction and the development of age-associated cognitive decline.9,10 Therefore, it is very important to prevent age- and sex-related decrease in cerebral blood flow.
Aerobic exercise training can affect arterial function. In previous studies, we and other groups have reported that aerobic exercise enhances arterial function.11–13 Furthermore, aerobic exercise training can improve cognitive function in older adults.14,15 Therefore, aerobic exercise training may have beneficial effects on the cardiovascular and cerebrovascular systems, e.g., prevention of cardiovascular and cerebrovascular diseases and dementia.16–18 Recently, a cross-sectional study demonstrated that exercise-trained men with high aerobic fitness exhibit higher cerebral blood flow velocity than their sedentary peers.19 However, the effects of aerobic exercise training on cerebral blood flow, especially in women, are unclear. Hence, a longitudinal study is required to clarify these effects.
The purpose of the present study was to examine the effects of exercise training on cerebral blood flow. We hypothesized that cerebral blood flow increases after aerobic exercise training in postmenopausal women. To test our hypothesis, we measured cerebral blood flow velocity and cerebrovascular resistance in postmenopausal female subjects before and after 8 weeks of moderate aerobic exercise training.
Methods
Subjects
A total of 20 healthy, sedentary postmenopausal women volunteered to participate in this study. They were assigned to either the exercise training group (n = 10) or the control group (n = 10). The subjects were nonsmokers, nonobese, and free of cardiovascular disease (assessed from the medical history). None of the subjects were taking cardiovascular-acting medications, including hormone replacement therapy. All subjects provided their written informed consent for participation. All the procedures were reviewed and approved by the ethical committee of the University of Tsukuba.
Exercise intervention
The subjects in the exercise training group underwent aerobic exercise training for 3–6 d/wk (2–3 supervised and additional home-based training) for 8 wk. Initially, they cycled and walked for 30 min/d at a relatively low exercise intensity (60% of their individual maximal heart rate). As their exercise tolerance improved, the duration of aerobic exercise was increased to 40–60 min/d, and the intensity to 70–75% of the maximal heart rate. The subjects in the control group were instructed not to change their physical activity level.
Measurements
All experiments were performed in the morning after a 12-h overnight fast. The subjects abstained from alcohol and caffeine for at least 12 h before the experiment and did not participate in any intensive exercise for a minimum of 24 h before starting the experiment. Measurements were obtained in a quiet temperature-controlled room (24–26 °C). After the subjects rested for at least 20 min, their cerebral blood flow velocity, cerebrovascular resistance, arterial blood pressure, and blood biochemistry parameters were analyzed. After obtaining these measurements, we also measured the aerobic capacity.
Cerebral blood flow
Cerebral blood flow velocities in the left and right middle cerebral arteries were measured using a 2-MHz pulsed Doppler ultrasonography system (EZ-Dop; DWL Elektronische System, Singen, Germany) as previously described.20 Doppler signals were obtained by adjusting the position for maximal reflected signal at a depth of 50–60 mm. Peak systolic, end-diastolic, and mean blood flow velocities were measured, and the pulsatility index was calculated by dividing the difference between peak systolic and end-diastolic velocities by the mean velocity. If flow velocity measurements were available for both sides, the average value was used. Beat-to-beat blood pressure was continuously measured by plethysmography (Portapres; FMS, Amsterdam, The Netherland) and calibrated using brachial blood pressure (Dinamap; GE Healthcare, Tokyo, Japan). The subjects were instructed to breathe normally and to avoid holding their breath. All transcranial Doppler (TCD) data were stored on a hard disk for off line analysis. The cerebrovascular resistance index was calculated by dividing the mean blood pressure by the mean blood flow velocity in the middle cerebral artery.
Blood biochemistry parameters
Blood samples were collected from the antecubital vein after an overnight fast. Serum concentrations of cholesterol and triglycerides and plasma concentrations of glucose were determined using standard enzymatic techniques.
Aerobic capacity
All the subjects underwent an incremental cycle exercise test (2 min at 40 W, followed by 20-W increments every 2 min) until 75% of the age-predicted maximal heart rate (220 – age in years) was reached. The heart rate (determined using a photoplethysmographic sensor) and workload (Watt) were recorded at the conclusion of each workload. The heart rate was regressed against workload and the resultant regression equation was used to calculate physical work capacity (Watt) at 75% of the age-predicted maximal heart rate (PWC75%). PWC has been shown to correlate well with directly measured maximal oxygen consumption.21
Statistical analysis
To determine the effect of each intervention on all outcome measures, repeated measures analysis of variance was used. When a significant main effect or interaction was observed, specific mean comparisons were performed to identify significance within each intervention. In the case of a significant T-value, a post-hoc test (Bonferroni test) was used to identify significant differences among mean values. All data have been reported as means ± SE. Statistical significance was set a priori at P < 0.05 for all comparisons.
Results
In the exercise training group, the average frequency and duration of exercise training were 4.1 ± 0.4 d/wk, and 47 ± 4 min/d, respectively. Table 1 shows select characteristics of the participants. Before the intervention, none of the variables significantly differed between the exercise training group and the control group. After the intervention, body weight and triglyceride levels significantly decreased, and high-density lipoprotein cholesterol level significantly increased in the exercise training group. PWC, an index of aerobic capacity, also increased significantly after the exercise training intervention. Heart rate and blood pressure did not change in both groups of subjects (Table 2).
| Control | Exercise training | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| n | 10 | 10 | ||
| Age, years | 61 ± 2 | – | 60 ± 2 | – |
| Height, cm | 155 ± 2 | – | 155 ± 1 | – |
| Body weight, kg | 51.3 ± 2.4 | 51.5 ± 2.4 | 55.7 ± 2.9 | 54.9 ± 2.9* |
| Total cholesterol, mmol/L | 5.8 ± 0.2 | 6.0 ± 0.2 | 5.6 ± 0.2 | 5.8 ± 0.2 |
| HDL cholesterol, mmol/L | 1.8 ± 0.1 | 1.9 ± 0.1 | 1.5 ± 0.1 | 1.8 ± 0.1* |
| LDL cholesterol, mmol/L | 3.5 ± 0.1 | 3.6 ± 0.1 | 3.6 ± 0.2 | 3.6 ± 0.2 |
| Triglyceride, mmol/L | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1* |
| Glucose, mmol/L | 4.9 ± 0.1 | 5.0 ± 0.1 | 4.9 ± 0.1 | 5.0 ± 0.1 |
| PWC75%, Watt | 48 ± 5 | 51 ± 6 | 63 ± 5 | 73 ± 6* |
Values are mean ± SE. HDL; high-density lipoprotein, LDL; low-density lipoprotein, PWC75%; physical work capacity corresponding to 75% of maximal heart rate.
P < 0.05 vs. before intervention.
Selected subjects characteristics.
| Control | Exercise training | |||
|---|---|---|---|---|
| Before | After | Before | After | |
| Heart rate and brachial blood pressure | ||||
| Heart rate, beats/min | 60 ± 1 | 60 ± 2 | 60 ± 2 | 60 ± 2 |
| Systolic blood pressure, mmHg | 112 ± 4 | 114 ± 4 | 117 ± 4 | 115 ± 4 |
| Diastolic blood pressure, mmHg | 69 ± 3 | 71 ± 3 | 72 ± 2 | 73 ± 2 |
| Mean arterial pressure, mmHg | 86 ± 3 | 90 ± 3 | 89 ± 3 | 91 ± 4 |
| Blood flow in meddle cerebral artery | ||||
| Peak systolic velocity, cm/s | 91 ± 5 | 93 ± 5 | 88 ± 6 | 99 ± 7* |
| End diastolic velocity, cm/s | 44 ± 3 | 44 ± 3 | 43 ± 3 | 50 ± 3* |
| Pulsatility index | 0.80 ± 0.03 | 0.80 ± 0.03 | 0.79 ± 0.02 | 0.75 ± 0.02 |
Values are mean ± SE.
P < 0.05 vs. before intervention.
Hemodynamic parameter before and after intervention.
No significant difference was observed in baseline blood flow velocity in the middle cerebral artery at rest between the exercise training and the control groups. A typical example of a subject’s cerebral blood flow velocity is given in Fig. 1. The pulsatility index of the middle cerebral artery did not change after the intervention (Table 2). The peak systolic and end-diastolic blood flow velocities significantly increased after exercise intervention (Table 2). The mean middle cerebral blood flow velocity significantly increased after exercise intervention (Fig. 2). Similarly, no significant difference was observed in baseline cerebrovascular resistance at rest, but cerebrovascular resistance significantly decreased after intervention in the exercise training group (Fig. 3).
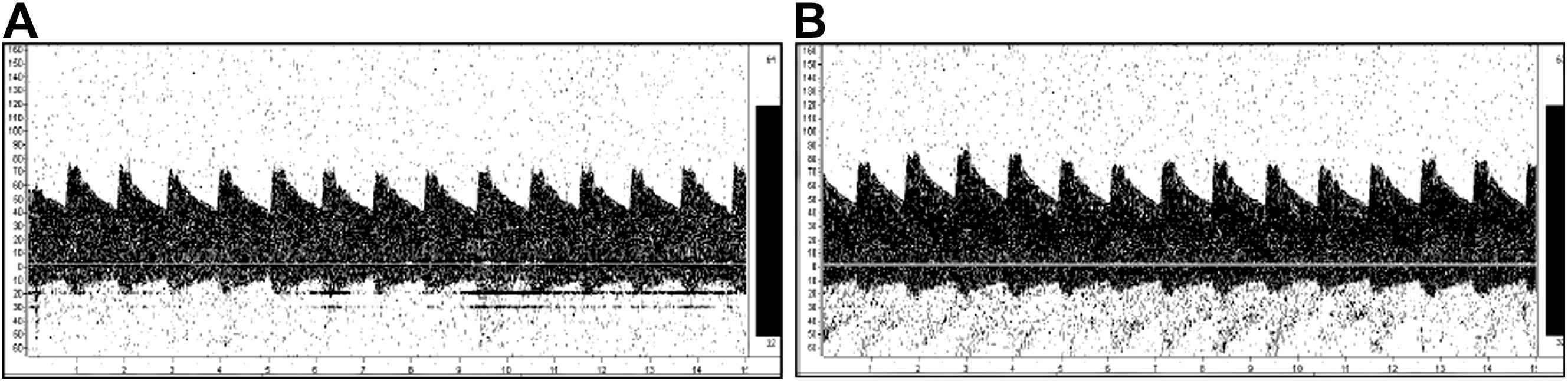
Typical transcranial Doppler signal before (A) and after (B) exercise intervention.
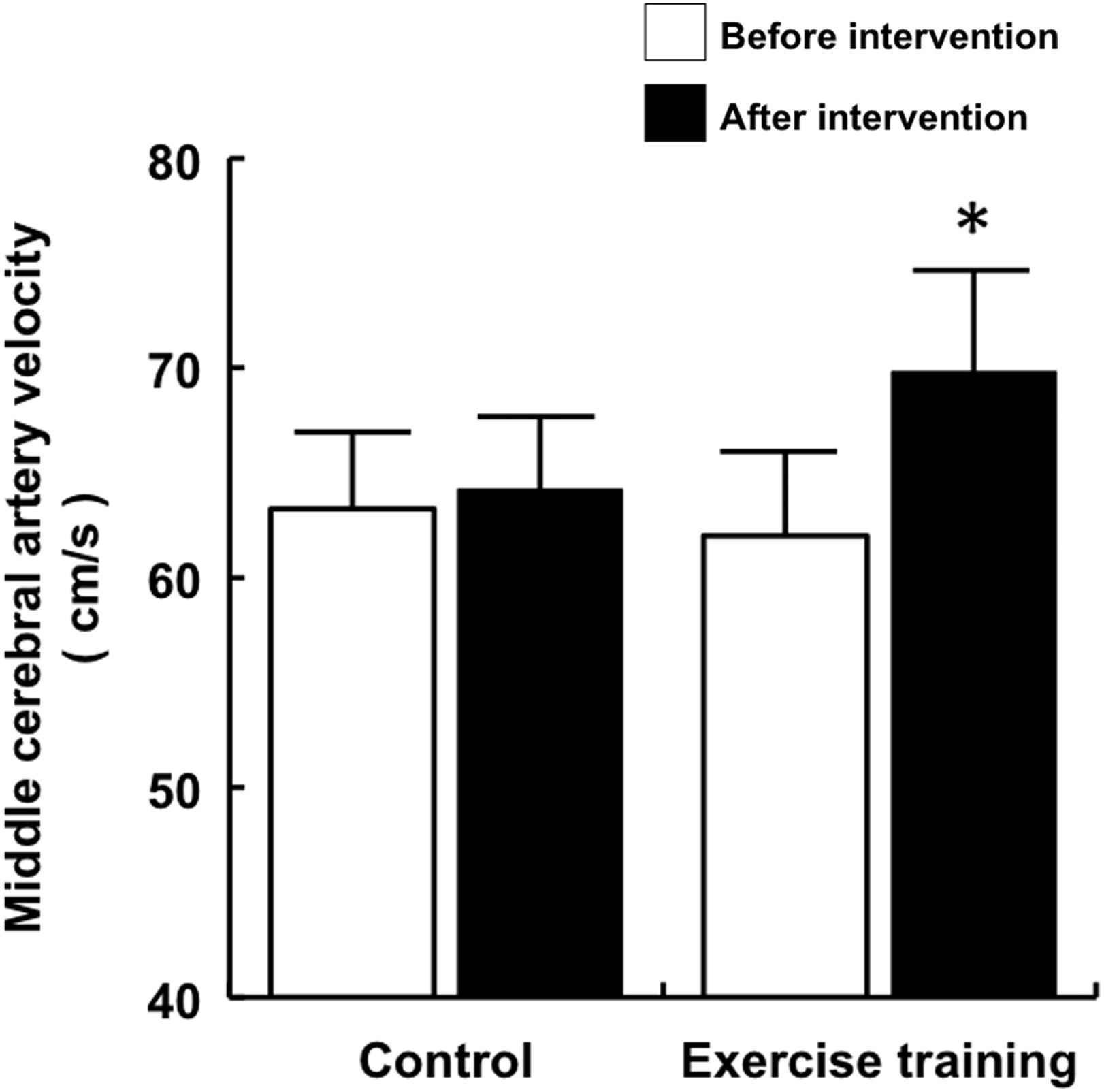
Cerebral blood flow velocity before and after intervention. Data are expressed as mean ± SE. *P < 0.05 vs. before intervention.
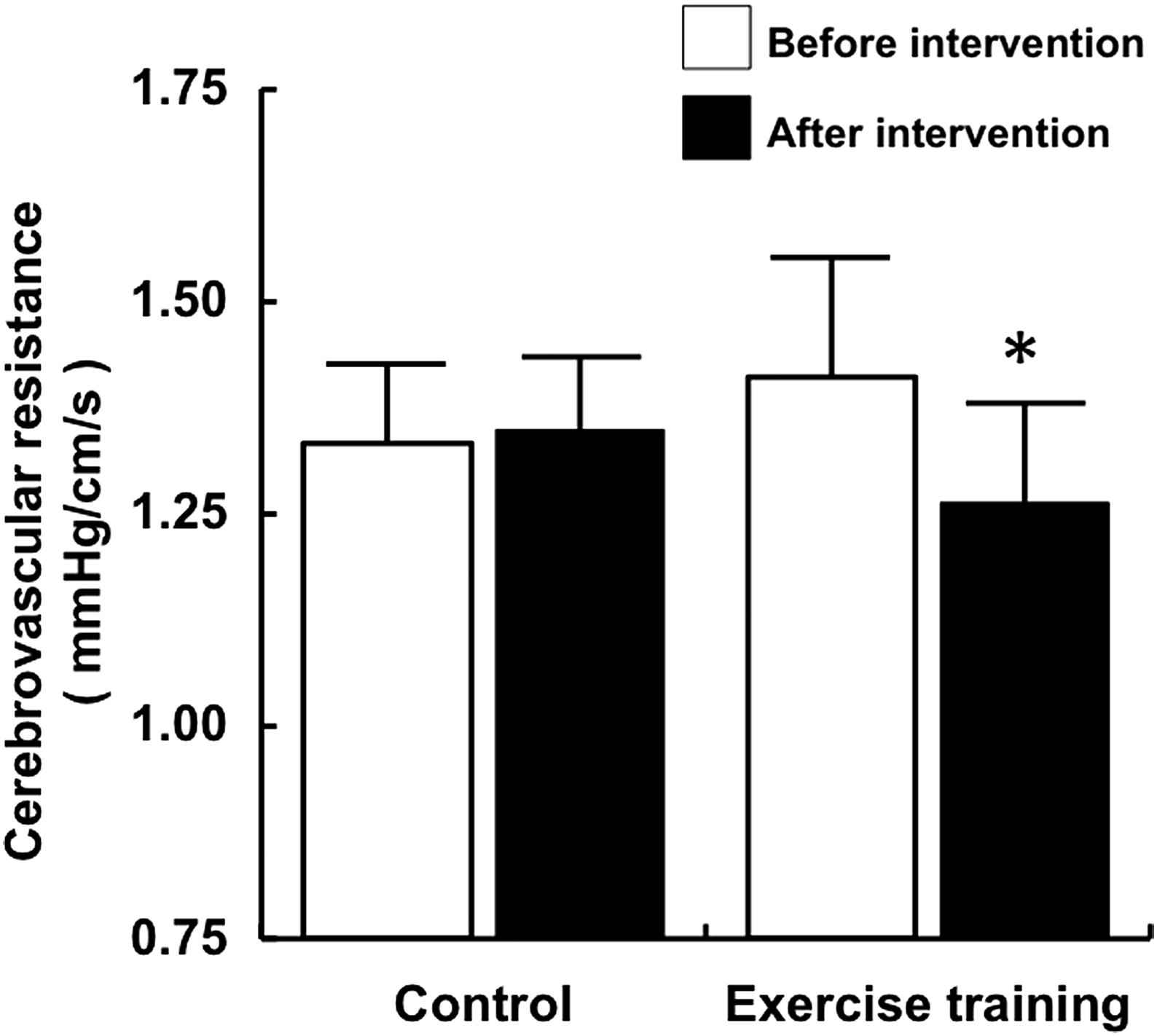
Cerebrovascular resistance before and after intervention. Data are expressed as mean ± SE. *P < 0.05 vs. before intervention.
Discussion
In this study, middle cerebral blood flow velocity increased and cerebrovascular resistance decreased after aerobic exercise training in postmenopausal women. We have thus provided the first direct evidence that aerobic exercise training increases cerebral blood flow in women and can therefore prevent age-related decreases in cerebral blood flow.
Decline in cerebral blood flow because of aging leads to neurodegeneration and apoptosis. Moreover, low cerebral blood flow is associated with global cerebral atrophy. To our knowledge, this study is the first to show, by longitudinal analysis, that aerobic exercise training increases the blood flow velocity in the middle cerebral artery. We evaluated this effect of aerobic exercise training by TCD analysis. Attenuation of middle cerebral artery velocity is a risk factor for cognitive decline and hippocampal and amygdalar atrophy in healthy subjects without dementia.8 In a cross-sectional study, Ainslie et al.19 suggested that regular aerobic-endurance exercise is associated with higher cerebral blood flow velocity in men aged 18–79 years. In this study, we longitudinally confirmed the favourable effect of aerobic exercise training on cerebral blood flow on the basis of the finding that cerebral blood flow velocity increased after aerobic exercise training. Therefore, aerobic exercise training may increase cerebral blood flow, which may contribute to inhibiting the pathogenesis of dementia.
The mechanism underlying the increase in blood flow velocity induced by aerobic exercise training in the middle cerebral artery remains to be elucidated. At least 2 possible mechanisms may be responsible: One mechanism is structural changes in the cerebral vessel. Aerobic activity in elderly subjects is associated with an increase in the number of small-calibre vessels, especially in the middle cerebral arterial region.22 Furthermore, animal studies have demonstrated that voluntary physical exercise improves angiogenesis in the brain and increases cerebral blood flow through increased endothelial nitric oxide (NO) systhase levels.23 Therefore, aerobic activity and/or aerobic exercise may increase cerebral blood flow owing to the changes in vessel structure. Another possible mechanism is functional change, that is, endothelial function may contribute to increasing cerebral blood flow velocity. Keilstein et al.24 demonstrated that infusion of asymmetrical dimethylarginine, an endogenous inhibitor of NO synthase, decreases cerebral perfusion. Several studies have reported that aerobic exercise training can improve age-related endothelial dysfunction,25,26 which is thought to be associated with enhancement of endothelial NO synthase.27 Faraci28 suggested that an increase in cerebral blood flow is largely mediated by endothelial NO synthase. Therefore, it is possible that the structural and functional changes in the cerebral vessels due to aerobic exercise may contribute to increasing cerebral blood flow velocity.
The present study has some limitations. First, we used TCD to measure cerebral blood flow velocity rather than cerebral blood flow. A previous study report indicated that the diameter of the middle cerebral artery, as measured by magnetic resonance imaging, did not change during a large change in cerebral flow velocity.29 Thus, it is likely that cerebral blood flow velocity provides a good estimate of cerebral blood flow. Furthermore, the Rotterdam study reported that cognitive decline is associated with the blood flow velocity of the middle cerebral artery.8 Secondly, we did not measure the arterial carbon dioxide tension and the end-tidal carbon dioxide tension (PETCO2). However, we analyzed the experimental reproducibility of the mean blood flow velocity in middle cerebral artery in the preliminary trial. The coefficient of variation (CV) of middle cerebral blood flow velocity and PETCO2 were 4.0% ± 0.5% and 1.1% ± 0.4% respectively, during the experimental procedure. The results of this study suggest that PETCO2 may not be related to blood flow in the supine rest position in this study. Thirdly, the small sample size of this study may be considered another limitation; however, the power calculation indicated that we had a sufficient number of subjects in the present study.
In conclusion, we demonstrated for the first time that aerobic exercise training increases blood flow velocity in the middle cerebral artery and decreases cerebrovascular resistance in postmenopausal women. Therefore, aerobic exercise training may attenuate age-related cerebral hypoperfusion in these women. Our findings may have important clinical implications for preventing cognitive decline and suggest that the increase in cerebral blood flow induced by aerobic exercise training may be one of the mechanisms underlying the preventive effect of this training on dementia.
Conflict of interest
We have no financial, consultant, institutional and other relationships that might lead to bias or a conflict of interest.
Acknowledgements
This work was supported by Grants-in-Aid for Scientific Research 21300234 and 21650179 from Japan Society for the Promotion of Science and the Ministry of Education, Culture, Sports, Science and Technology (MEXT) for the Body and Mind Integrated Science Project (2010–2013)
References
Cite this article
TY - JOUR AU - Nobuhiko Akazawa AU - Youngju Choi AU - Asako Miyaki AU - Jun Sugawara AU - Ryuichi Ajisaka AU - Seiji Maeda PY - 2012 DA - 2012/06/07 TI - Aerobic exercise training increases cerebral blood flow in postmenopausal women JO - Artery Research SP - 124 EP - 129 VL - 6 IS - 3 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2012.05.003 DO - 10.1016/j.artres.2012.05.003 ID - Akazawa2012 ER -
