Interrelationships between macro and microvascular structure and function
- DOI
- 10.1016/j.artres.2010.10.001How to use a DOI?
- Keywords
- Antihypertensive therapy; Macrocirculation; Microcirculation
- Abstract
Macrovasculature and microvasculature are interrelated, and may influence each other. Microvascular structure is not only the site of vascular resistance but probably also the origin of most of the wave reflections generating increased central systolic blood pressure. Some index of large artery stiffness seems to be related with the media to lumen ratio of subcutaneous small resistance arteries of hypertensive patients. In addition, both microvascular structural alterations and changes in the mechanical properties of large arteries represent potent predictors of prognosis. In addition, drugs that may improve microvascular structure may also correct alterations in the mechanical properties of large arteries In fact, while β-blocking agents and diuretics have a negligible effect on microvascular structure, renin-angiotensin system antagonists and calcium entry blockers have favorable actions, improving also large artery mechanics and possibly reducing central wave reflections.
- Copyright
- © 2010 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Mechanical properties of large arteries in hypertension
Arterial stiffness plays a key role in the pathophysiology of the cardiovascular system.1 Arterial compliance favours left ventricular function as it reduces left ventricular workload, and enhances diastolic perfusion, crucial to the delivery of blood to the myocardium through the coronary vessels. It is possible to measure arterial stiffness from the carotid to femoral PWV (normal values: <12 m/s).1,2 Pressure wave can reflect from the peripheral vasculature (branching, resistance sites, stenosis), and return towards the heart.1 When stiffness is high, the returned wave may add to the ejection pressure. In physiological conditions, the reflected pressure wave returns in diastole, explaining why the systolic and pulse pressures measured close to the heart (central blood pressure) are lower than at the periphery.3
Age and blood pressure are the two major determinants of increased arterial stiffness.1 Molecular determinants of arterial stiffness are related to the fibrotic components of the extracellular matrix, mainly elastin, collagen and fibronectin. Increased arterial stiffness was consistently observed in conditions such as hypertension, dyslipidemia and diabetes.1 As blood vessels become stiffer because of age-related processes, the pulse wave is transmitted more rapidly and returns to the heart during left ventricular contraction, resulting in a greater augmentation of the central aortic systolic pressure. It is therefore possible to quantify this effect through the calculation of the augmentation index (ratio of augmented pressure to pulse pressure; augmented pressure:height of the late systolic peak above the inflection).2
Alterations in the mechanical properties of large arteries have a clear pathophysiological link with clinical outcome. In particular, carotid–femoral PWV yielded prognostic values beyond and above traditional risk factors.2 In addition, increased aortic augmentation index is associated with coronary artery disease.3 Central pressures also correlate with cardiovascular risk not only in patients with atherosclerotic disease but also in apparently healthy subjects. The late systolic augmentation of the central pressure waveform is associated with an increase in left ventricular mass index independent of age and mean blood pressure3 and carotid systolic blood pressure is an independent determinant of left ventricular wall thickness. Moreover, central pressure is also more closely related than brachial pressure to other important cardiovascular intermediate end points, such as vascular hypertrophy, extent of carotid atherosclerosis, and ascending aorta diameter.3
Microvascular structure and function in hypertension
Resistance arteries are key elements in the control of blood pressure. The main drop in hydrostatic pressure occurs at the level of resistance arteries; i.e. small resistance arteries (<300 μm of lumen diameter), arterioles (<100 μm of lumen diameter) and capillaries (about 7 μm of lumen diameter).4 Thus, structural changes in the microcirculation may directly and strongly affect blood pressure values. In fact, it is now widely accepted that structural abnormalities of microvessels are commonly associated with chronic hypertension.4,5 The majority of the available data indicates that, in patients with essential hypertension, the resistance arteries show a greater media thickness, a reduced internal and external diameter with increased media to lumen ratio, without any significant change of the total amount of wall tissue, as indicated by an unchanged media cross-sectional area. Therefore, the major part of the structural changes observed in these patients is the consequence of inward eutrophic remodeling (re-arrangement of the same amount of wall material around a smaller vessel lumen),6,7 without net cell growth. In contrast, in patients with some forms of secondary hypertension the development of hypertrophic remodeling with a more evident contribution of vascular smooth muscle cell hypertrophy was observed.5
Alterations in the microcirculation are often associated with and may also play an important role in the development of organ damage in hypertension. A significant correlation between coronary flow reserve and subcutaneous small resistance artery remodeling was detected in hypertensive patients, thus suggesting that structural alterations in small resistance arteries may be simultaneously present in different vascular districts and that changes in morphology of the subcutaneous vasculature may even reflect clinically more important alterations in the coronary vessels.8 Thus, the presence of structural alterations in the microcirculation may be considered an important link between hypertension and ischemic heart disease, heart failure, cerebral ischemic attacks, and renal failure. We have previously demonstrated that structural alterations in the microcirculation (as indicated by greater values of media to lumen ratio in subcutaneous small resistance arteries) are probably the most powerful predictor of cardiovascular events in a high-risk population of hypertensive patients.9 In this study the relative importance of each prognostic factor, adjusted for the others, was assessed using Cox’s proportional hazard model. Microvascular structure and pulse pressure (a rough index of large artery distensibility) were the only two predictors of cardiovascular events, even when all traditional risk factors were included in the model,9 thus suggesting that, in these high-risk subjects, structural changes in the microcirculation and alterations on mechanical properties of large arteries are the two most important factors in predicting outcome.
Relationships between structural changes in the microcirculation and macrocirculation
Due to the elastic properties of large arteries, the pulsatile pressure and flow that result from intermittent ventricular ejection transformed in a continuous flow, so that microvasculature mediates steadily the delivery of nutrients and oxygen to tissues.10 The disruption of this function, which occurs when microvascular structural alterations develop in response to hypertension, leads to end-organ damage. Microvascular structure is not only the site of vascular resistance but also, probably, the origin of most of the wave reflections generating increased central systolic blood pressure, especially in the elderly patients,10 although the proper location of a reflection site is elusive.11 We have investigated possible relationships between subcutaneous small resistance artery structure, and blood pressure values in a population of normotensive subjects and hypertensive patients. Among the most important predictors of small artery structure there were clinic systolic, diastolic and mean blood pressure and the ratio between pulse pressure and stroke volume, taken as an index of large artery distensibility.10,12 We also investigated possible relationships between indices of large artery distensibility and media to lumen ratio of subcutaneous small resistance arteries. We have observed relatively strong relationships between PWV (Fig. 1), brachial pulse pressure (r = 0.42, p < 0.05) and systolic pressure (r = 0.35, p < 0.05) and media to lumen ratio, thus suggesting that structural alterations in large and small vessels may be reciprocally interrelated. Large artery stiffening was also demonstrated to be related to cerebral lacunar infarctions, which are usually expression of cerebral microvascular disease.13 Recently, Katsi et al.14 have observed, in hypertensive patients, a progressive stiffening of the aorta (carotid–femoral PWV) in parallel with the evolution of the fundus oculi vascular lesions according to Scheie’s scale. The authors concluded that probably the same pathophysiological processes, i.e. endothelial function impairment or structural remodeling occur simultaneously in small and in large vessels in hypertension.14
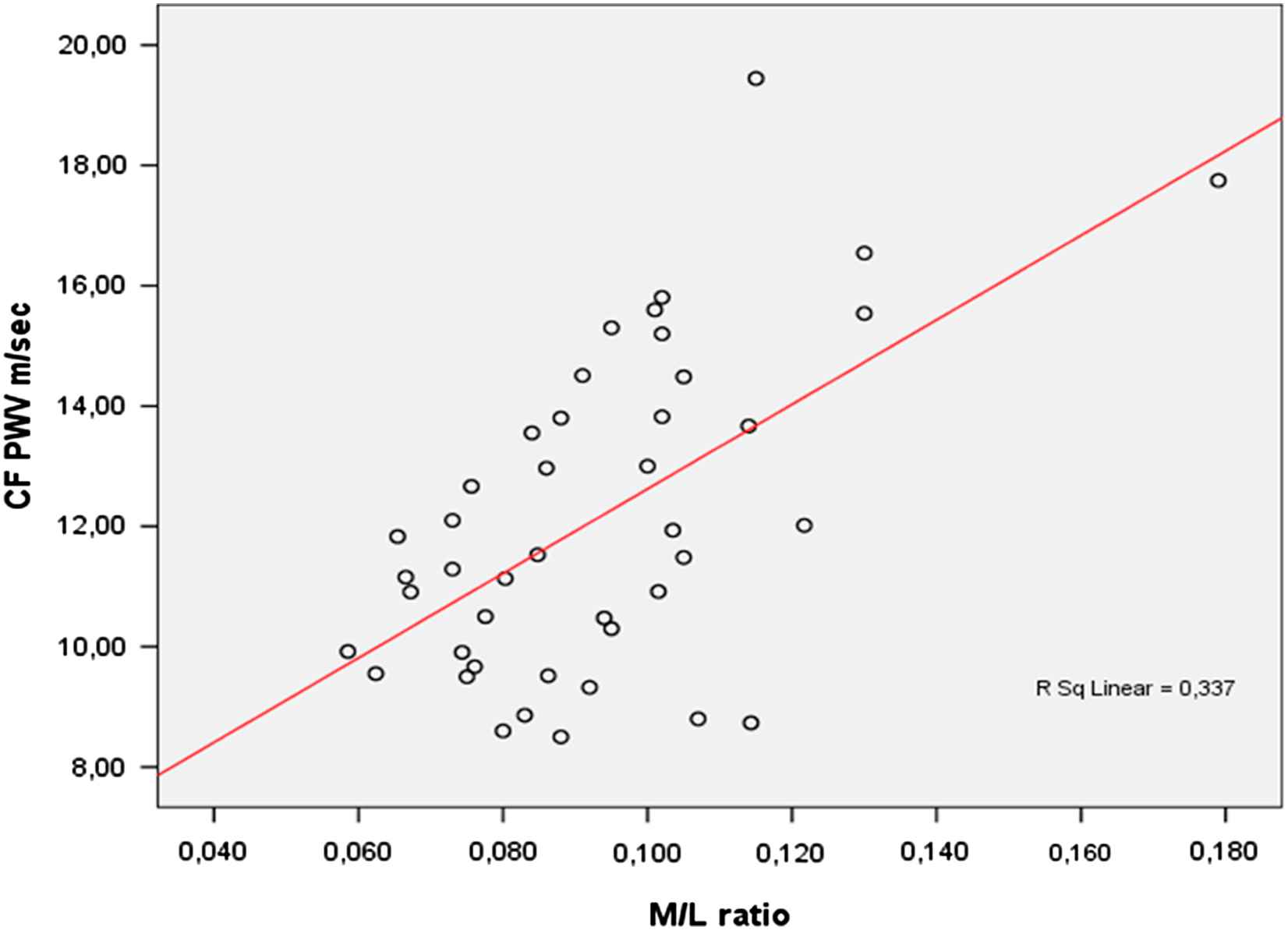
Correlation between carotid to femoral pulse wave velocity (CF PWV) and media to lumen ratio of subcutaneous small resistance arteries (M/L ratio): r = 0.58, p < 0.05. N = 43 patients (8 never treated), 21 males and 22 females, age range 31–65 years, 22 essential hypertensive patients at high cardiovascular risk (10 with type 2 diabetes mellitus), 11 patients with primary aldosteronism, 6 patients with pheochromocytoma, 4 obese patients (unpublished data). The relationship remained statistically significant even after correction for the effects of age, serum glucose and systolic arterial pressure (r = 0.37, p = 0.04).
Additional interrelationships between micro and macrovascular structure are mediated by possible microvascular consequences of atherosclerotic alterations in large arteries.15 A hemodynamically relevant stenosis can be compensated through lowering downstream vascular resistance.16 This reduces the amplitude of the flow reserve, so that if needed, flow reserve may not be sufficient for adequate supply of the end organ, which may in turn lead to clinical symptoms. Also, repeated microembolisms may alter downstream vascular resistance.17 On the other hand, microvasculature may also influence the function of upstream larger arteries. Coronary microangiopathy is associated with a reduction of upstream dilatatory function of epicardial coronary arteries,18 in great part due to development of endothelial dysfunction and decreased production of nitric oxide (NO). Microvascular dysfunction with a reduced flow reserve may lead to a reduced shear stress-induced NO synthesis in upstream macrovessels.19
Effects of antihypertensive treatment
Some intervention studies have demonstrated an improvement or even an almost complete normalization of the structure of subcutaneous small resistance arteries with angiotensin converting enzyme (ACE) inhibitors (cilazapril, perindopril, lisinopril),20 calcium channel blockers (nifedipine, amlodipine, isradipine),20 angiotensin II receptor blockers (losartan, irbesartan, candesartan and valsartan).20 On the contrary, the β-blocker atenolol and the diuretic hydrochlorothiazide were devoid of effects on resistance vessels and on brachial pulse pressure, despite a blood pressure reduction similar to that observed with ACE inhibitors.20 In one of these studies, performed in hypertensive patients with non-insulin dependent diabetes mellitus,21 brachial pulse pressure was significantly higher in patients than in normotensive subjects before random assignment and it was significantly reduced only by the angiotensin AT1 receptor antagonist valsartan, while no change was observed with atenolol. Brachial pulse pressure may be considered a marker of arterial stiffness in the presence of preserved ejection fraction.10 Stiffness of small arteries (evaluated by the stress–strain relationships) was, in fact, enhanced in patients treated with atenolol, and a similar change may have also occurred in large arteries.21 This may explain differences in brachial systolic blood pressure and also different effects of the drugs on small artery remodeling at the end of the study.
The effects of antihypertensive treatment with ACE inhibitors on arterial stiffness and wave reflections were investigated by evaluation of brachial and carotid systolic blood pressure and pulse pressure (indirect indices of arterial stiffness) in the REASON Study (preterax in REgression of Arterial Stiffness in a contrOlled double-bliNd study)22 and further confirmed.10 The REASON Study was a controlled trial that compared the β-blocking agent atenolol to a low-dose combination of the diuretic indapamide with the ACE inhibitor perindopril. After 1 year, for the same diastolic blood pressure reduction, the combination of perindopril and indapamide induced a more pronounced reduction of brachial (peripheral artery) and carotid (central artery) systolic blood pressure or pulse pressure than atenolol.22 The two drug regimens induced the same aortic PWV reduction as a result of the comparable decreases of mean or diastolic arterial pressures.10 The major difference between the two therapeutic approaches was that perindopril/indapamide but not atenolol reduced carotid augmentation index, a reliable marker of carotid wave reflections.10,23 Several mechanisms may be involved in the observed differences between the two therapeutic approaches. The atenolol-induced heart rate decrease may have caused the maintenance of disturbed wave reflections,10,23 since it has been previously demonstrated that a slow heart rate can also affect PWV and augmentation of central aortic systolic pressure.10,23 It is possible that perindopril, but not atenolol, may have improved micro and macrovascular structure. In addition, ACE inhibitors, but not atenolol, are known to reduce reflection coefficients,10,23 thus reducing the reflection of waves from the microcirculation toward large arteries. In the CAFE study (Conduit Artery Function Evaluation),24 the different effects of atenolol/hydrochlorothiazide and amlodipine/perindopril on central blood pressure accounted probably for a significant part of the different effect on cardiovascular outcome observed in the ASCOT trial (Anglo-Scandinavian Cardiac Outcomes Trial), in which an advantage of the combination ACE inhibitor–calcium channel blocker was clearly observed. A more pronounced and favourable effect of calcium channel blockers, diuretics and ACE inhibitors compared with β-blockers on central aortic systolic blood pressure or central augmentation pressure was also observed by Morgan et al.25
In conclusion, cardiovascular events are the consequence of vascular damage at both the macro and microcirculatory level. Interrelationships between alterations in the macro and microcirculation and mechanisms possibly involved represent an extremely interesting topic, which deserve further and thorough investigation, also considering its relevant clinic impact in terms of possible prevention or regression of the related alterations.
References
Cite this article
TY - JOUR AU - Damiano Rizzoni AU - Maria Lorenza Muiesan AU - Enzo Porteri AU - Carolina De Ciuceis AU - Gianluca E.M. Boari AU - Massimo Salvetti AU - Anna Paini AU - Enrico Agabiti Rosei PY - 2010 DA - 2010/10/29 TI - Interrelationships between macro and microvascular structure and function JO - Artery Research SP - 114 EP - 117 VL - 4 IS - 4 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2010.10.001 DO - 10.1016/j.artres.2010.10.001 ID - Rizzoni2010 ER -
