A cardiovascular phenotype in warfarin-resistant Vkorc1 mutant rats☆
Grant support: Hamill Innovation grant; and National Heart, Lung, and Blood Institute (NHLBI).
- DOI
- 10.1016/j.artres.2008.09.002How to use a DOI?
- Keywords
- Aorta; Mineralization; Renal artery; Urolith; Vitamin K epoxide reductase; Warfarin
- Abstract
Background: The inhibition of the vitamin K cycle by warfarin promotes arterial calcification in the rat. Conceivably, genetically determined vitamin K deficiency owing to a mutant epoxide reductase subcomponent 1 (Vkorc1) gene, a key component of the vitamin K cycle, might also promote arterial calcification. In the absence of an available Vkorc1 gene knockout model we used a wild-derived Vkorc1 mutant rat strain (Rattus norvegicus) to explore the validity of this hypothesis.
Methods: We provide histopathological descriptions of a naturally occurring Vkorc1 gene knockdown: wild-derived lab-reared rats that are resistant to the anticoagulant warfarin owing to a non-synonymous mutation in the Vkorc1 gene (Vkorc1Y→C), which, in vitro, reduces the basal activity of the vitamin K epoxide reductase enzyme complex by ~52%. H&E stained sections of heart and kidney were compared between homozygous Vkorc1Y→C/Y→C, heterozygous Vkorc1Y→C/+ and wildtype Vkorc1+/+ rats of both sexes.
Results: We observed that the aorta of the heart was mineralized in the Vkorc1Y→C/Y→C male rats but lesions were virtually absent from Vkorc1Y→C/+ and Vkorc1+/+ male and all female rats. The renal arteries were mineralized in Vkorc1Y→C/Y→C and Vkorc1Y→C/+ mutant rats, regardless of sex.
Conclusions: Results support a hypothesis that posits that Vkorc1 genetic polymorphisms reducing basal enzyme activity could affect cardiovascular health, with dependencies on genotype, sex, and tissue. The undercarboxylation of the vitamin K-dependent Matrix Gla protein may be the crucial component of the pathway promoting this mineralization.
- Copyright
- © 2008 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
The understanding of the chemical nature of vitamin K antagonists and their connections to the vitamin K cycle, as well as the long history spanning the efforts of the biochemical purification, genetic mapping, and molecular cloning of the vitamin K 2,3-epoxide reductase protein complex VKOR,1–10 have culminated in the identification of a gene, now named vitamin K epoxide reductase subunit 1, Vkorc1.11,12 The Vkorc1 encodes a warfarin-sensitive protein that could in fact be the key enzyme, or rather, part of the key enzyme complex VKOR, of the vitamin K cycle.13 The gene appears to be responsible for vitamin K epoxide reduction to the active vitamin KH2 cofactor for the γ-carboxylase. The γ-carboxylase is responsible for the post-translational γ-carboxylation of vitamin K-dependent proteins, notably blood coagulation factors and Matrix Gla protein MGP.14,15
The vitamin K cycle is appreciated for its role in blood coagulation.14,16 In this context the vitamin K cycle is of therapeutic relevance because it can be inhibited by the oral anticoagulant drug warfarin.15 This application of warfarin is an area where the value of personalized medicine is under investigation by analyzing Vkorc1 genetic polymorphisms to predict warfarin dosing in human study cohorts.17–20 Mutations in the Vkorc1 gene were found to be associated with a rare severe bleeding phenotype in humans.21
The vitamin K cycle may mediate processes more generally related to cardiovascular health.14,23–28 For example, inhibition of the VKOR by warfarin in the extrahepatic tissues of laboratory rats resulted in the mineralization of the aorta.29–31 Inhibition of the VKOR influences downstream processes with dependencies on vitamin K as a cofactor for the γ-carboxylation of proteins, notably MGP.27,32 MGP is an important inhibitor of vessel and cartilage calcification that is expressed in human calcified atherosclerotic plaques.33–40 Arterial calcification could be accelerated by oral anticoagulant therapy, as mediated by the inhibition of the VKOR by warfarin and the resulting undercarboxylation of MGP.20,41 Genetic polymorphisms in the MGP have been shown to affect cardiovascular health.42 Thus, conceivably, genetic polymorphisms in the Vkorc1 gene also might affect cardiovascular health.43
Warfarin is also used as a rodent poison.16 Numerous wild populations of rodents have evolved resistant to warfarin.44 In rat Rattus norvegicus populations in Germany, where our wild-derived rats were collected, there is near complete association between a mutated Vkorc1 and warfarin resistance, which led the authors to propose that the Vkorc1 mutation forms the molecular genetic basis of warfarin resistance.22,45 Warfarin resistance is under natural selection in free-living rat populations because the carriers of the mutation are at an advantage over the wildtype on farms and in townships where warfarin is used.46–49 However, this advantage of resistance may be offset by some pleiotropic fitness cost related to vitamin K deficiency.50,51 Typically, this cost is thought to be due to vitamin K deficiency and lethal hemorrhage restricted to the homozygous mutant rats.52 This scenario lead to the conclusion that warfarin resistance generally is under overdominant natural selection, i.e., both the warfarin-resistant and warfarin-susceptible homozygous genotypes have lower fitness relative to the warfarin-resistant heterozygous genotype.47,53,54
Here we examined warfarin-resistant rats for the phenotypic effects of a naturally occurring gene knockdown mutation in the Vkorc1, and thus, we bypass the current lack of a Vkorc1 gene knockout model. We tested the hypothesis that warfarin resistance might have consequences to vitamin K-dependent processes that are independent of the effects on blood coagulation. The central argument is that the recycling of vitamin K in mutant strains resistant to the vitamin K antagonist warfarin is itself inefficient due to the altered Vkorc1, leading to a relative vitamin K-deficient state in the warfarin-resistant rat compared to wildtype rats. This relative vitamin K deficiency should lead to an undercarboxylation of vitamin K-dependent proteins, notably the potent inhibitor of vascular mineralization MGP, thereby promoting vessel calcification.
Methods
Rat strains
This study was conducted on rats sampled from an area in Northwestern Germany.22,45,55,56 Rats from this area carry a non-synonymous mutation in the Vkorc1 gene from A to G, resulting in the tyrosine to cysteine (Y–C) amino-acid change at position 139 in exon 3. This amino-acid change results in a 47% reduction of the basal in vitro Vkorc1 activity.11,22,57 The Vkorc1 genotypes are designated as Vkorc1Y→C/Y→C (homozygous mutant), Vkorc1Y→C/+ (heterozygous mutant) and Vkorc1+/+ (wildtype). The strain examined is resistant to the anticoagulant warfarin if it is Vkorc1Y→C/Y→C or Vkorc1Y→C/+, i.e. the mutation is dominant with respect to warfarin resistance (but is of incomplete penetrance58).
Rats were trapped in the field and were kept in the laboratory for the months specified as age in supplementary Table 1 (which thus is an underestimate of the age of those rats), or rats were born by wild-caught rats in the laboratory and were of the given age in supplementary Table 1. Rats were kept in Macrolon® cages on shavings from saw mills with tap water and standard lab food (Altromin® 1324) ad lib. The diet contained 3 mg/kg Vitamin K3. The Institutional Animal Care and Use Committee approved the study.
The warfarin-resistant (RW) and warfarin-susceptible (RW+) phenotypes were distinguished by using a blood clotting response test (BCR) that measured percent clotting activities (PCA) after injection of a diagnostic dose of warfarin22,55 (c.f. Rodenticide Resistance Action Committee 2003, a reappraisal of blood clotting response tests for anticoagulant resistance and a proposal for a standardized BCR test methodology: Technical Monograph, Crop. Life International). Male and female rats were induced with 7 mg/kg or 8.5 mg/kg warfarin, respectively, by intraperitoneal injection, which corresponds to 4 multiples of the ED50 in susceptible rats. This treatment unlikely explains the systematic differences among genotypes described in the results section as all genotypes were subjected to it. The presence of the A–G mutation at position 139 in exon 3 of the Vkorc1 gene was confirmed by PCR as described.22
Histology
The aorta and kidney were harvested and preserved by immersion in 10% buffered formalin. Tissues underwent conventional processing and resulting paraffin sections were stained with hematoxylin and eosin (H&E). Specialized stains for calcium with Von Kossa and Alizarin red were repeated multiple times with no positive result. The diagnosis of mineralization in this exploration study was, therefore, made entirely based on the characteristic appearance of the mineralization after H&E staining. The cause of the inability to confirm the presence of mineral in the tissue using special staining techniques was not determined, but there clearly is a need to specifically identify the chemical nature of this mineralization during future analyses. We note that our rats showed diffuse signs of postmortem autolysis because they went through freeze–thaw cycles during transport. However, it is not clear how this could have resulted in systematic differences in mineralization between Vkorc1 genotypes during our study. Slides listed in supplementary Table 1 are available from M.H.K. for inspection.
Initially, evaluation of histological sections focused on the presence or absence of mineralization as judged by R.E.P during a blind test, i.e., in the absence of information on sex, phenotype, and genotype. Subsequently, histological sections were reviewed with respect to potential miss-classifications during the blind test. During this informed secondary review a fuller description of the nature and degree of lesions was compiled. Finally, to improve our ability to evaluate the best model describing the data and to gauge the importance of potentially worrisome parameters (see below), we attached arbitrarily scaled numerical ordinal scores to the degree of mineralization: 0 for absence and 1, 2, and 3–4 for minimal, modest, and marked levels of mineralization, respectively.
Statistical analysis
Nominal logistic regression as implemented in the J
Results
Aorta
A cardiovascular phenotype detected by H&E staining on histological sections was manifested as mineralization in the aorta of the heart and of the branching arteries, usually found to be focal to multifocal coalescing. It typically occurred in the adventitia of the aorta and in the tunica media and adventitia at the intersection of the branching artery with the aorta (Figs. 1 and 2).
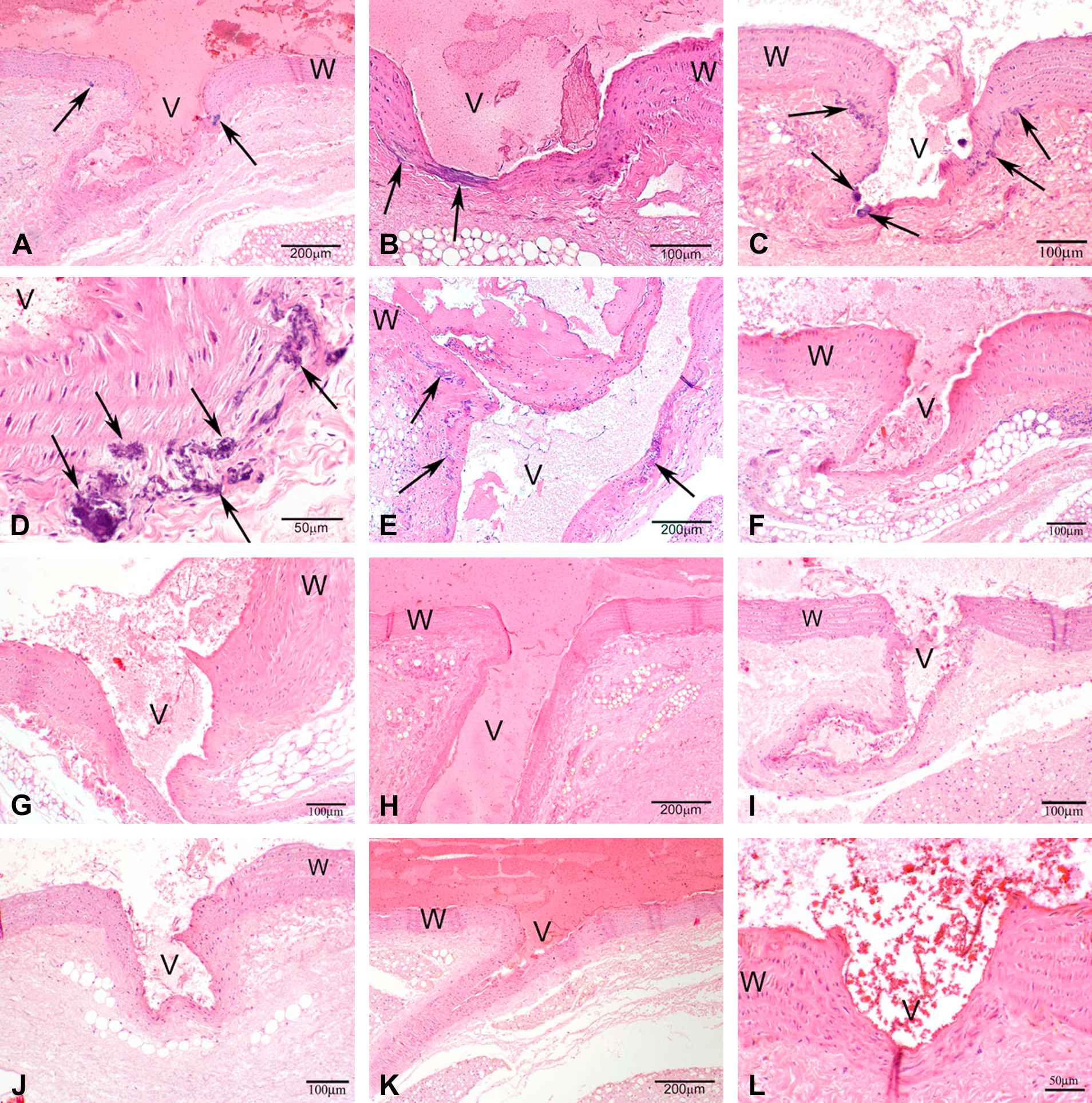
Photomicrographs of H&E stained sections under 40× magnification (50 μm resolution) of histological sections of the aorta from male rats. (A–E) Vkorc1Y→C/Y→C warfarin-resistant male rats. (F–H) Vkorc1Y→C/+ heterozygous warfarin-resistant rats. (I–L) Vkorc1+/+ warfarin-susceptible rats. V = lumen, W = wall of aorta. C.f. supplementary Table 1 for full description of slides.

Photomicrographs of H&E stained sections under 40× magnification (50 μm resolution) of histological sections of the aorta from female rats. (A–D) Vkorc1Y→C/Y→C warfarin-resistant female rats. (E–F) Vkorc1Y→C/+ heterozygous warfarin-resistant rats. (G–I) Vkorc1+/+ warfarin-susceptible rats. C.f. legend to Fig. 1.
Effect of warfarin-resistance phenotype and gender
Mineralization was found present in 4 of the 8 and 5 of the 8 warfarin-resistant (RW) male rats during the blind test and after secondary review, respectively (Table 1). All 4 warfarin-susceptible (RW+) males were classified as free of mineralization during the blind test and secondary review. Mineralization was absent or minimal in RW and RW+ females (Table 1).
| Sex | Phenotypes and genotypes | Present | Absent | Description | Average score (range) | Total |
|---|---|---|---|---|---|---|
| Males | RW | 4/5 | 4/3 | None to marked lesions | 1.1 (0–3) | 8 |
| Vkorc1Y→C/Y→C | 4/5 | 1/0 | Minimal to marked lesions | 1.8 (1–3) | 5 | |
| Vkorc1Y→C/+ | 0/0 | 3/3 | No lesions | 0 | 3 | |
| RW+ (Vkorc1+/+) | 0/0 | 4/4 | No lesions | 0 | 4 | |
| Subtotals (N=) | 4/5 | 8/7 | – | – | 12 | |
| Females | RW | 0/2 | 6/4 | None to minimal lesions | 0.3 (0–1) | 6 |
| Vkorc1Y→C/Y→C | 0/2 | 4/2 | None to minimal lesions | 0.5 (0–1) | 4 | |
| Vkorc1Y→C/+ | 0/0 | 2/2 | No lesions | 0 | 2 | |
| RW+ (Vkorc1+/+) | 0/0 | 3/3 | No lesions | 0 | 3 | |
| Subtotals (N=) | 0/2 | 9/7 | – | – | 9 |
RW and RW+ designate the homozygous (Vkorc1Y→C/Y→C) and heterozygous (Vkorc1Y→C/+) warfarin-resistant and warfarin-susceptible phenotype, respectively.
Scoring of mineralization as present or absent in histological sections of the male and female aorta during blind test (first number) and after secondary review (second number)
Effect of warfarin-resistance genotype and gender
Four out of 5 Vkorc1Y→C/Y→C male rats displayed signs of mineralization during the blind test, and all 5 Vkorc1Y→C/Y→C male rats displayed minimal to marked signs of mineralization after secondary review (Table 1). In contrast, none of the 3 Vkorc1Y→C/+ and 4 Vkorc1+/+ males and all females were judged free of mineralization. However, 2 Vkorc1Y→C/Y→C female rats displayed minimal signs of mineralization during secondary review (Table 1).
A statistical model incorporating the parameters sex, genotype and their interaction term explained ~79% of the variation in the response variable ‘presence or absence’ of mineralization (nominal logistic regression, χ2 = 21.2, d.f.: 5, p = 0.0007). We were concerned that the worrisome parameters origin and age of rats would have an effect on results. We conducted stepwise regression on a prior model that incorporated all parameters, including age and origin, as well as the possible interaction terms. Only sex (p = 0.003), genotype (p < 0.001) and their interaction term (p = 0.007) were significant. The model incorporating these parameters was significant (ANOVA, R2 = 75.1%, d.f.: 3, Fratio: 17.5, p < 0.0001). Age (p = 0.712) and origin (p = 0.452) were not significant individually or when part of an interaction term.
Overall, male sex and warfarin-resistance genotype primarily determine the mineralization of the aorta in our rat strain, and the Vkorc1 mutation appears to be recessive with respect to the mineralization of the aorta (Fig. 3).
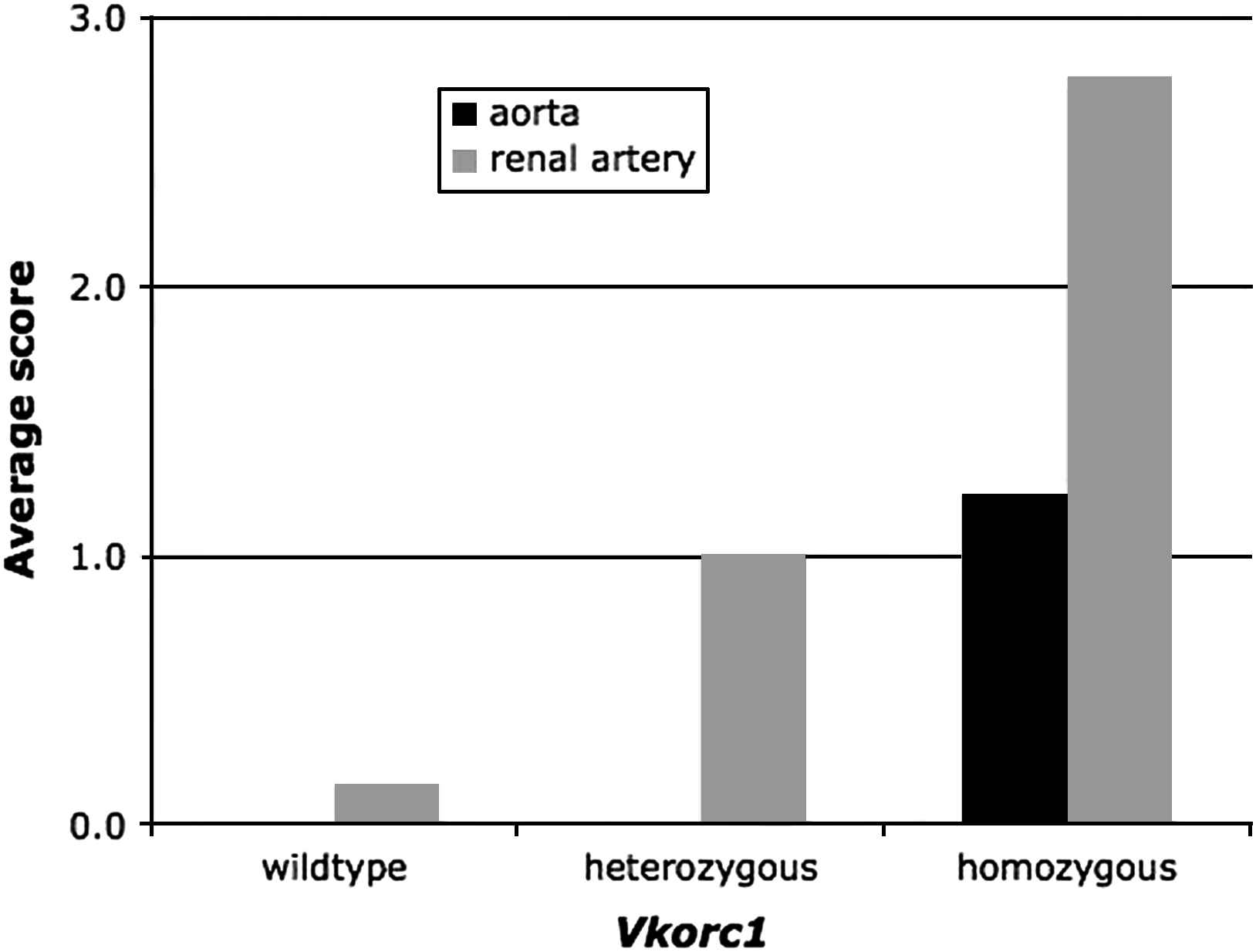
The average score assigned to the degree of mineralization of the aorta and of the renal arteries of male and female rats in the warfarin-resistant and warfarin-susceptible Vkorc1 mutant genotypes. Note that the scaling of scores was arbitrary and in relation to slides of the same tissue only, such that the score between aorta and renal arteries, though shown side-by-side here, should not be directly compared. Note also that the average score of the mineralization of the aorta in homozygous female rats was 0.5 whereas in males the score was 1.8 (see text).
Renal arteries
Warfarin-resistance phenotype and genotype determined the mineralization of the renal arteries of the kidney, however, a sex-specific effect was not detected (Fig. 3). If present, the mineralization generally was multifocal coalescing of the endothelial surface of the renal arteries exiting the kidney (predominantly in the heterozygous genotype) and, the renal arteries inside the kidney in some samples with full mineralization of the arterial wall (predominantly in the homozygous mutant genotype) (Figs. 4 and 5). Uroliths were seen in 3 female rats, 2 of which were Vkorc1Y→C/+ and 1 was Vkorc1Y→C/Y→C (c.f. Fig. 4).
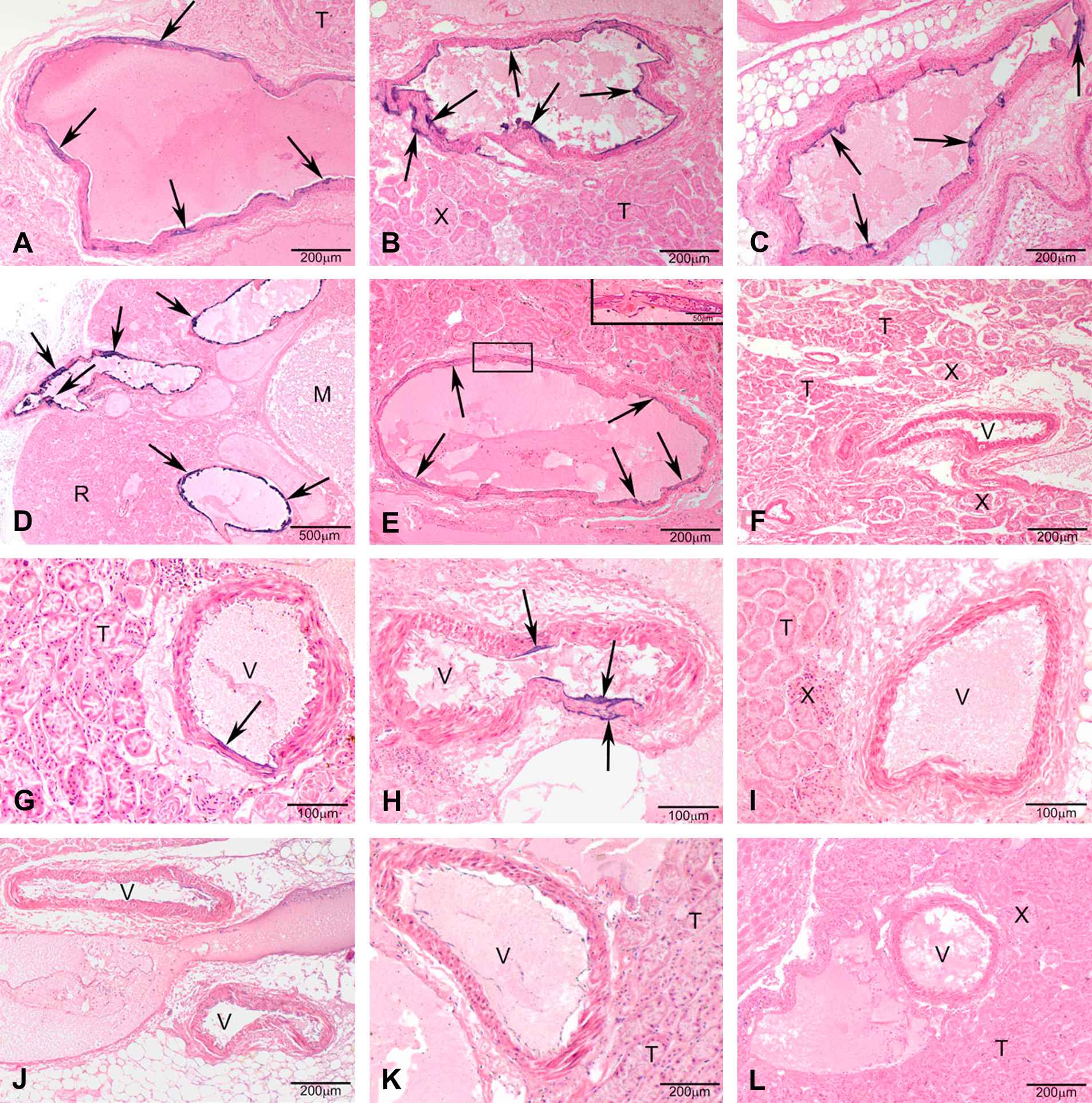
Photomicrographs of H&E stained sections under 40× magnification (50 μm resolution) of histological sections of the renal arteries from male rats. (A–E) Vkorc1Y→C/Y→C warfarin-resistant male rats. (F–H) Vkorc1Y→C/+ heterozygous warfarin-resistant rats. (I–L) Vkorc1+/+ warfarin-susceptible rats. Arrows depict mineralization. T = tubules, V = lumen of the renal arteries, M = macula, X = juxtaglomerula. C.f. supplementary Table 1 for full description of slides.
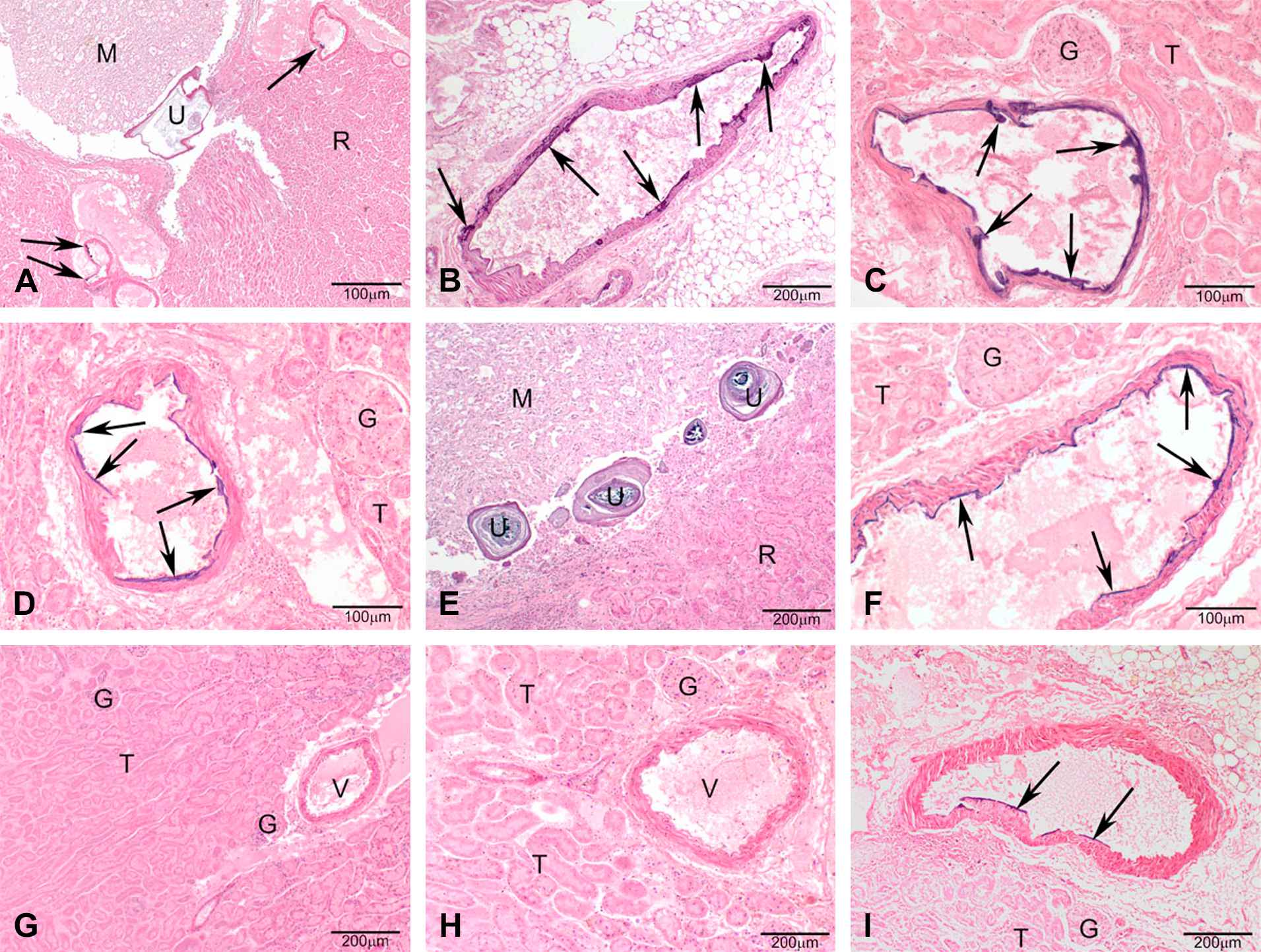
Photomicrographs of H&E stained sections under 40× magnification (50 μm resolution) of histological sections of the renal arteries from female rats. (A–D) Vkorc1Y→C/Y→C warfarin-resistant female rats. (E–F) Vkorc1Y→C/+ heterozygous warfarin-resistant rats. (G–I) Vkorc1+/+ warfarin-susceptible rats. C.f. legend to Fig. 4.
Effect of warfarin-resistance phenotype and gender
Mineralization was assessed as present in 6 of the 8 and 7 of the 8 RW male rats during the blind test and after secondary review, respectively, (Table 2). In contrast, all 4 RW+ males were free of signs of mineralization. Mineralization was also found present in all 6 and 5 of the 6 RW female rats during the blind test and after secondary review, respectively (Table 2). All 3 RW+ females were free of signs of mineralization or, upon secondary review, 1 of the 3 was judged to display minimal lesions.
| Sex | Phenotypes and genotypes | Present | Absent | Description | Average score (range) | Total |
|---|---|---|---|---|---|---|
| Males | RW | 6/7 | 2/1 | None to marked lesions | 2.4 (0–4) | 8 |
| Vkorc1Y→C/Y→C | 5/5 | 0/0 | Moderate to marked lesions | 3.2 (2–4) | 5 | |
| Vkorc1Y→C/+ | 1/2 | 2/1 | None to moderate lesions | 1.0 (0–2) | 3 | |
| RW+ (Vkorc1+/+) | 0/0 | 4/4 | No lesions | 0 | 4 | |
| Subtotals (N=) | 6/7 | 6/5 | – | – | 12 | |
| Females | RW | 6/5 | 0/1 | None to marked lesions | 1.8 (0–3) | 6 |
| Vkorc1Y→C/Y→C | 4/4 | 0/0 | Moderate to marked lesions | 2.3 (2–3) | 4 | |
| Vkorc1Y→C/+ | 2/1 | 0/1 | None to moderate lesions | 1.0 (0–2) | 2 | |
| RW+ (Vkorc1+/+) | 0/1 | 3/2 | None to minimal lesions | 0.3 (0–1) | 3 | |
| Subtotals (N=) | 6/6 | 3/3 | – | – | 9 |
C.f. legends to Table 1.
Scoring of mineralization as present or absent in histological sections of the male and female renal arteries
Effect of warfarin-resistance genotype and gender
All 5 RW male rats with the Vkorc1Y→C/Y→C mutation displayed moderate to marked signs of mineralization (Table 2). Of the 3 Vkorc1Y→C/+ males 1 and 2 showed signs of mineralization during the blind test and after secondary review, respectively. After secondary review 2 warfarin-resistant female rats homozygous for the Vkorc1Y→C/Y→C mutation were judged to display minimal signs of mineralization (Table 2).
A statistical model incorporating genotype explained ~55% of the variation in the response variable ‘presence or absence’ of mineralization (nominal logistic regression, χ2 = 15.4, d.f.: 2, p = 0.0004). In addition, stepwise regression on a prior model that incorporated all parameters and interaction terms showed that only genotype (p < 0.0001) and age (p = 0.014) emerged as significant parameters. The model incorporating these was significant (ANOVA, R2 = 77.4%, d.f.: 2, Fratio: 30.8, p < 0.0001). Importantly, a comparison of the Vkorc1Y→C/Y→C and Vkorc1Y→C/+ genotypes, which did not differ in age (p = 0.303, Wilcoxon/Kruskal–Wallis Rank Sums Test) showed that these were significantly different in the mineralization (Fig. 3, p = 0.012, Wilcoxon/Kruskal–Wallis Rank Sums Test), suggesting age is not a parameter that can explain the mineralization fully.
Overall, warfarin-resistance phenotype explains the degree of mineralization of the renal arteries well, and the Vkorc1Y→C mutation is co-dominant (Fig. 3). The effect of age merits closer investigation during future studies.
Discussion
In this pilot study we reported on a cardiovascular phenotype in wild-derived warfarin-resistant rats from Germany that carry an A–G mutation at position 416 in the Vkorc1 transcript, resulting in a Y–C amino-acid change at position 139 in the protein.11,22,45,57 The genotypes Vkorc1Y→C/Y→C, Vkorc1Y→C/+, both warfarin-resistant, RW, and the warfarin-susceptible (RW+) genotype Vkorc1+/+ were compared for their cardiovascular phenotypes (Tables 1 and 2). The reason for using wild-derived warfarin-resistant rats having the Vkorc1 mutations is the lack of an available Vkorc1 gene knockout in an inbred rodent strain, which would have been a better experiment.
The phenotypes examined were concerned with the mineralization of the aorta and of the renal arteries as deduced from H&E stained histological sections. We observed mineralization of the aorta predominantly in the homozygous warfarin-resistant male rats. Mineralization in the renal arteries appeared in both sexes almost exclusively in warfarin-resistant genotypes. The finding of uroliths is in agreement with the expression of vitamin K-dependent proteins, including MGP, in urolithiasis,59 but owing to the small number of observations should be interpreted with caution.
We suggest that the observed phenotypes are due to a ~53% reduction of the basal VKOR enzyme in vitro activity in the Vkorc1Y→C/Y→C genotype compared to the Vkorc1+/+ genotype.11,22,45,57 Conceivably, the Vkorc1Y→C/+ mutant rat should have an ~25% reduced VKOR activity, i.e., the mutation is co-dominant with respect to VKOR activity in the warfarin-unchallenged state. This may be a valid assumption, because in the warfarin-challenged state the homozygous mutant rat has ~87% of in vitro VKOR activity while the heterozygote has about half of that (~42%), suggesting warfarin-resistant alleles are co-dominant with incomplete penetrance.58
Our results support the hypothesis that warfarin resistance in rats might have physiological consequences to vitamin K-dependent processes that are independent of the effects on blood coagulation. Specifically, the recycling of vitamin K in warfarin-resistant rats is itself inefficient due to the altered Vkorc1 subunit, leading to relative vitamin K deficiency in the warfarin-unchallenged state. This relative vitamin K deficiency is expected to result in the undercarboxylation of vitamin K-dependent proteins that play a role in arterial calcification, foremost MGP.
To confirm vitamin K deficiency in vivo future work should measure VKOR activity in individual rats in the warfarin-unchallenged state. Moreover, to obtain a good measure for vitamin K recycling capacity it would be desirable to measure the hepatic- and/or blood vitamin K 2,3-epoxide to vitamin K ratio. To implicate the MGP pathway in the pleiotropic effects of warfarin resistance histochemical measures of carboxylated and undercarboxylated MGP are needed.23 In general, a more exhaustive phenotyping of the Vkorc1 mutant rat during future studies seems to be warranted now. Finally, to eliminate concerns that the observed pattern is due to genetic background our study should be replicated in strains where only the Vkorc1 gene carrying a mutation has been introgressed into laboratory strains.
Our results should be interpreted with respect to the particular mutation and strain under study. First, owing to the outbred genetic background of rats we caution that genetic polymorphisms linked to the Vkorc1, or in downstream biochemical pathways, may have contributed to the observed effects. It is known that strain-specific modifier loci, some sex-specific, affect the warfarin resistance profile58,60–62 and, conceivably, other phenotypes, including the mineralization of the cardiovascular system. Second, it is possible that other mutations in the Vkorc1 gene differ in their expression of the cardiovascular phenotypes. Finally, it should be noted that for our rat strain from Germany dietary vitamin K requirements were not found to be measurably elevated during a pilot survey (H-J Pelz, pers. observation). The functional elimination of the VKOR might boost the up-regulation of other pathways for the recruitment of vitamin KH2, resulting in the maintenance of hepatic levels of vitamin K needed to maintain blood coagulation.55 However, our study is inconsistent with the ability of such pathways, if applicable, to compensate for extrahepatic vitamin K deficiency resulting in the cardiovascular phenotypes.
With the caveats mentioned above in mind our study should be of interest to the biomedical field by showing a plausible histological cardiovascular phenotype associated with a Vkorc1 polymorphism. Support for such an association has relied on human studies. Given the complex genetic architecture of cardiovascular health and environmental influences in human additional evidence from a rodent model was needed. Our study now provides such support for an association of Vkorc1 mutations and cardiovascular mineralization. Assuming that our results are portable to the human condition, we suggest that genetic variation in the Vkorc1, and subtle variations in vitamin K cycling over the lifetime of human subjects, are factoring into the mineralization of the aorta and of the renal arteries.
Disclosures
The authors confirm that there is no conflict of interest to disclose.
Acknowledgements
This work was funded in part by a Hamill innovation grant to MHK and Michael Liebschner from the Institute of Biosciences and Bioengineering, Rice University and by grant R01 HL091007 from the National Heart, Lung, and Blood Institute (NHLBI) to MHK. We are thankful for our discussions with Michael Liebschner and for the comments made by anonymous reviewers.
Appendix A
Supplementary material
Supplementary material can be found, in the online version, at doi:
References
Cite this article
TY - JOUR AU - Michael H. Kohn AU - Roger E. Price AU - Hans-Joachim Pelz PY - 2008 DA - 2008/11/07 TI - A cardiovascular phenotype in warfarin-resistant Vkorc1 mutant rats☆ JO - Artery Research SP - 138 EP - 147 VL - 2 IS - 4 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2008.09.002 DO - 10.1016/j.artres.2008.09.002 ID - Kohn2008 ER -
