Implication of bone regulatory factors in human coronary artery calcification
- DOI
- 10.1016/j.artres.2011.06.003How to use a DOI?
- Keywords
- Bone; Coronary; Artery; Calcification
- Abstract
Background: Although emerging evidence suggests that vascular calcification constitutes an active process sharing common features with bone formation, several aspects of this process in human coronary artery calcification are still poorly understood. We therefore investigated the expression of key bone regulatory factors in human atherosclerotic coronary arteries.
Methods – Results: Formalin, fixed-paraffin embedded tissue samples of human atherosclerotic coronary arteries (n = 41) and normal arteries as controls (n = 9) were studied immunohistochemically for the expression of osteoprotegerin (OPG), RANKL, RANK, Runx2, Sox9, NFATc1 and Osterix (Osx). All factors where expressed in atherosclerotic lesions while absent in normal arteries, with the exception of OPG. While expression of NFATc1 and Osx was confined to tunica intima of diseased arteries, the others factors were expressed in both tunica intima and tunica media. Most factors were expressed in smooth muscle-like cells of tunica intima while NFATc1 and the OPG/RANKL/RANK system were also expressed in inflammatory cells. Wheareas expression of OPG and RANKL was invariable, expression of RANK, Runx2, Sox9, Osx and NFATc1 was significantly higher in advanced calcified lesions. Significant correlations were also observed among the bone regulatory factors in atherosclerotic arteries.
Conclusions: Our results confirm the hypothesis that highly regulated osteogenic processes are involved in the mineralization of human coronary arteries and implicate the bone regulatory factors Osx and NFATc1 in coronary artery calcification.
- Copyright
- © 2011 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Arterial calcification is a significant risk factor for the occurrence and complications of atherosclerotic disease. Calcification can increase the risk of plaque rupture and is associated with a significant increase in cardiovascular mortality.1 Although previously considered degenerative, it is now becoming evident that arterial calcium mineral deposition is an active, cell-mediated and highly regulated process2 with resemblance to bone remodeling.3,4 Indeed, calcified arteries and cultured vascular cells have been shown to express bone matrix proteins and factors known to regulate osteoblastic and chondrocytic differentiation.5,6
Receptor activator of nuclear factor-kB ligand (RANKL), a member of the tumor necrosis factor superfamily, is the principal regulator of osteoclast biology.7 RANKL through activation of its receptor RANK, promotes osteoclast differentiation, activation and survival,8 while osteoprotegerin (OPG), a secreted tumor necrosis receptor factor homolog, increases bone mineral density by acting as a decoy receptor for RANKL.9,10 Besides regulating bone mass, OPG/RANKL/RANK axis is essential for immune responses and it also seems to exert important effects on the vascular system through both immunomodulatory and osteogenesis-related mechanisms.11,12
Runx2/Cbfa1 belongs to the Runt-domain family and is a master bone regulatory factor inducing transcription of osteoblast-related genes that enhance mineralization.13–16 The SRY-related transcription factor Sox9 regulates the expression of chondrocyte-specific genes and functions upstream of Runx2 in chondrocyte differentiation and bone formation.17–19 Recently, Osterix (Osx), a zinc finger-transcription factor, was shown to be required for osteoblast differentiation acting downstream of Runx2.20,21 Runx2 and Osx activity is considered indispensable for intramembranous and endochondral bone formation.22
Nuclear factor of activated T cells c1 (NFATc1), a transcriptional factor that belongs to the Rel Group, is considered to be a major regulator of T cell maturation and osteoclast differentiation.23,24 Increasing evidence suggests that NFATc1 also exerts major effects in the transcriptional program of osteoblasts.25 Interestingly, NFATc1 is related to both RANKL/RANK and Osx signaling during bone remodeling.25,26
Recent data show that the OPG/RANKL/RANK system, as well as the osteogenic and chondrogenic regulatory factors Runx2 and Sox9 are involved in the pathobiology of vascular calcification.6,11 Mice lacking OPG showed severe osteoporosis and arterial calcification providing the first evidence that the OPG/RANKL/RANKL system could be an important autocrine/paracrine axis in vascular calcification.27–29 Increased expression of Runx2 and Sox9 has been also demonstrated in cultured human vascular cells as well as in calcified regions of human atherosclerotic vessels.6,30 However, our understanding of their specific roles in human coronary arterial calcification remains limited. In addition, despite the well established osteo-regulatory functions of Osx and NFATc1, their role in arterial calcification is poorly understood. We therefore investigated using immunohistochemistry the expression of OPG, RANKL, RANK, Runx2, Sox9 as well as Osx and NFATc1 in human coronary arteries. Correlations with cardiovascular risk factors and severity of atherosclerotic lesions were also examined.
Material and methods
Study group
The study was performed in accordance with ethical guidelines and has been approved by the Committee on Research and Ethics and the Scientific Committee of the University Hospital of Patras, Greece.
Current study was performed on fifty human subjects (n = 50) who had died from non-cardiac causes such as suicide, trauma or accident. Sample material derived from nine subjects (n = 9) was normal and was used as control. The mean age of patients with atherosclerotic arteries was 47 (range 16–81). Thirty six (n = 36) were men and five (n = 5) were women. The mean age of control patients was 27 (range 14–38). Seven (n = 7) were men and two (n = 2) were women. None of them had undertaken osteoporosis treatment in the past. Clinical records were reviewed for cardiovascular risk factors and treatment regimen (Table 1).
| Patients | Control Donors | p value | |
|---|---|---|---|
| Total number, n (%) | 41 (100) | 9 (100) | |
| Age; mean (range) | 47 (16−81) | 27 (14–38) | 0.532 |
| Sex M/F, n (%) | 36 (88)/5 (12) | 7 (78)/2 (22) | 0.655 |
| Body mass index, n (%) | <0.05 | ||
| <18.5 | 0 (0) | 3 (33.3) | |
| 18.5–24.9 | 10 (24) | 3 (33.3) | |
| 25–29.9 | 28 (69) | 3 (33.3) | |
| >30 | 3 (7) | 0 (0) | |
| Coronary Artery Disease, n (%) | 10 (24.4) | 0 (0) | 0.101 |
| Smoking, n (%) | 23 (56) | 3 (33) | 0.220 |
| Diabetes Mellitus, n (%) | 3 (7.3) | 0 | 0.407 |
| Hypertension, n (%) | 21 (51) | 1 (11) | <0.05 |
| Dyslipidemia, n (%) | 10 (24.4) | 1 (11) | 0.389 |
| Family History of Coronary Artery Disease, n (%) | 7 (17) | 0 (0) | 0.186 |
| Chronic renal failure, n (%) | 1 (2.4) | 0 (0) | 0.639 |
| Statins, n (%) | 10 (24.4) | 1 (11) | 0.389 |
| ACE inhibitors, n (%) | 21 (51) | 1 (11) | <0.05 |
| Beta–blockers, n (%) | 12 (29.2) | 0 (0) | 0.065 |
| Calcium channel Blockers, n (%) | 3 (7.3) | 0 (0) | 0.407 |
| Loop diuretics, n (%) | 8 (19.5) | 0 (0) | 0.152 |
ACE: angiotensin converting enzyme.
Patients and control demographics.
Coronary artery disease was defined as a positive history of myocardial infarction, typical angina, myocardial revascularization or a positive stress testing.
Smoking was defined as regular daily cigarette smoking or having stopped in the previous 12 months.
Diabetes Mellitus (DM) was defined as use of an oral hypoglycaemic agent or insulin, fasting glucose >126 mg/dL or post-challenge glucose >200 mg/dL (75 g oral glucose tolerance test).
Hypertension was defined as systolic blood pressure >140 mm Hg, diastolic blood pressure >90 mm Hg, or use of anti-hypertensive medication.
Dyslipidemia was defined as an HDL concentration <45 mg/dl in men or <55 mg/dl in women, a total cholesterol concentration ≥200 mg/dl, an LDL concentration ≥100 mg/dl, or a fasting triglyceride concentration ≥200 mg/dl or as having ever been prescribed medication for high cholesterol.
Family history of CAD was defined as 1st-degree relatives (parents, brothers and/or sisters) with myocardial infarction before age 60 or stroke at any age.
Chronic renal failure was defined as chronic renal supportive therapy (i.e., chronic hemodialysis, hemofiltration, or peritoneal dialysis) for irreversible renal disease or history of chronic renal insufficiency associated with clinical adverse effects (usually creatinine >300 μmol/L).
Tissue specimens
Formalin-fixed, paraffin-embedded tissue samples of fifty coronary arteries (n = 50) were obtained at autopsy. Proximal three centimeters of left anterior descending artery (LAD) were opened longitudinally and under proper light source were examined for every possible atherosclerotic lesion. Subsequent sampling was performed. Several studies provide evidence that proximal portion of LAD comprises the most frequent location of atherosclerotic lesions.31–33 Atherosclerotic lesions were identified in forty one patients (n = 41). Atheromatous lesions were classified as stages 1, 2 and 3 according to their severity. Stage 1 included type I and II lesions (n = 12), stage 2 included type III and IV lesions (n = 13) and stage 3 included type Vb calcified lesions (n = 16) according to the classification of the American Heart Association Committee.34
Immunohistochemistry
Immunohistochemistry was performed as previously described35 using specific antibodies for OPG, RANKL, RANK, Runx2, Sox9, Osx and NFATc1. For negative controls, blocking solution was added instead of the primary antibody. Antibody characteristics, dilutions, incubation time and pre-treatment conditions are shown in Table 2 .
| Antibody | Type | Source | Antigen retrieval | Protein blocking | Dilution | Incubation time |
|---|---|---|---|---|---|---|
| OPG | P* | Santa Cruz, CA, USA | 0.01M citrate buffer | PBS – 1.5% BSA | 1:20 | 2 h, RT |
| RANKL | P* | Santa Cruz, CA, USA | 0.01M citrate buffer | PBS – 1.5% BSA | 1:30 | 2 h, RT |
| RANK | P* | Santa Cruz, CA, USA | 0.01M citrate buffer | PBS – 1.5% BSA | 1:20 | 2 h, RT |
| Runx2 | M | Novus Biologicals, Littleton, CO, USA | 0.01M citrate buffer | TBS – 10% milk | 1:100 | 1 h, RT |
| Sox9 | P | Santa Cruz, CA, USA | 0.01M citrate buffer | TBS – 3% BSA | 1:100 | ON, 4° C |
| Osterix | P | Abcam, Cambridge, UK | – | TBS – 3% BSA | 1:20 | 1 h, RT |
| NFATc1 | M | Santa Cruz, CA, USA | 1M EDTA, pH= 8.0 | TBS – 3% BSA | 1:25 | ON, 4° C |
P: Polyclonal goat; P: Polyclonal rabbit; M: Monoclonal mouse; PBS: Phosphate buffered saline; TBS: Tris-buffered saline; BSA: Bovine serum albumin; ON: Overnight; RT: Room temperature.
Antibody characteristics.
Immunohistocemical evaluation
All slides were assessed by two pathologists (HP, JV) and one investigator (AA) independently and blinded to the case.
Immunohistochemical staining was evaluated based on the percentage of positive cells as follows: 0: <10% of cells positive; 1: 10%–35% positive cells, 2: expression in 35%–70% of cells, 3: immunoreactivity in >70% of cells.
Statistical analysis
Correlations of protein expression with the stage of atherosclerotic lesions and clinical parameters were assessed with the non-parametric Mann–Whitney and Kruskal–Wallis tests for ordinal data. Correlations between expressions of proteins were analyzed by the Spearman rank–order correlation coefficient because variables were not normally distributed and sample size was small. The significance level was defined as p < 0.05. All statistical analysis was performed with the SPSS for Windows, release 12.0 (SPSS inc, Chicago, IL, USA).
Results
There was a positive staining for OPG in normal arteries confined to smooth muscle cells of tunica media (Fig. 1A) while RANKL and RANK expression was absent in control specimens (Fig. 1C, E). In atherosclerotic coronary arteries OPG cytoplasmic staining was noticed mostly in smooth muscle-like cells and to a lesser extent in macrophages of tunica intima (Fig. 1B) and in smooth muscle cells of tunica media. Immunoreactivity for RANKL and RANK in diseased arteries was observed in smooth muscle-like cells and inflammatory cells of tunica intima (Fig. 1D, F) as well as in smooth muscle cells of tunica media. While there was no significant difference in OPG expression levels among the different stages of atherosclerotic lesions (Table 3), expression of RANK in both tunica intima and tunica media was significantly higher in advanced atherosclerotic lesions (p = 0.004) (Table 3). Expression of RANK in tunica intima of atherosclerotic arteries correlated with the expression of RANKL (p = 0.047, r = 0.316). There was no association of OPG, RANKL and RANK expression levels with cardiovascular risk factors.

Immunoreactivity of OPG/RANKL/RANK system in human coronary arteries. Normal arteries showing positive expression of OPG in smooth muscle cells (A) in contrast to the negative staining of RANKL (C) and RANK (E). Representative cases of human atherosclerotic coronary lesions showing positive OPG expression in smooth muscle-like cells and inflammatory cells near atherosclerotic lesions (B) and intense RANKL (D) and RANK (F) expression. (×400).
| Left coronary artery atherosclerosis | |||||
|---|---|---|---|---|---|
| Total | IHC score | I–II | III–IV | Vb | p value |
| 12 | 13 | 16 | |||
| n (%) | n (%) | n (%) | |||
| OPG expression | 0 | 4 (33.3) | 3 (23.1) | 8 (50.0) | p = 0.54 |
| 1 | 5 (41.7) | 2 (15.4) | 1 (6.25) | ||
| 2 | 3 (25.0) | 7 (53.8) | 5 (31.25) | ||
| 3 | 0 (0) | 1 (7.7) | 2 (12.5) | ||
| RANKL expression | 0 | 1 (8.3) | 1 (7.7) | 0 (0) | p = 0.109 |
| 1 | 4 (33.3) | 2 (15.4) | 2 (12.5) | ||
| 2 | 5 (41.7) | 8 (61.5) | 7 (43.75) | ||
| 3 | 2 (16.7) | 2 (15.4) | 7 (43.75) | ||
| RANK expression | 0 | 1 (8.3) | 0 (0) | 0 (0) | p = 0.014 |
| 1 | 1 (8.3) | 2 (15.4) | 1 (6.25) | ||
| 2 | 8 (66.7) | 3 (23.0) | 3 (18.75) | ||
| 3 | 2 (16.7) | 8 (61.6) | 12 (75.0) | ||
| Runx2 expression | 0 | 10 (83.3) | 5 (38.5) | 1 (6.25) | p < 0.001 |
| 1 | 2 (16.7) | 7 (53.8) | 6 (37.25) | ||
| 2 | 0 (0) | 1 (7.7) | 4 (25.0) | ||
| 3 | 0 (0) | 0 (0) | 5 (31.25) | ||
| Sox9 expression | 0 | 1 (8.3) | 1 (7.7) | 0 (0) | p < 0.001 |
| 1 | 10 (83.3) | 0 (0) | 2 (12.5) | ||
| 2 | 0 (0) | 7 (53.8) | 4 (25.0) | ||
| 3 | 1 (8.3) | 5 (38.5) | 10 (62.5) | ||
| Osterix expression | 0 | 12 (100) | 7 (53.8) | 1 (6.25) | p < 0.001 |
| 1 | 0 (0) | 4 (30.8) | 6 (37.25) | ||
| 2 | 0 (0) | 2 (15.4) | 6 (37.25) | ||
| 3 | 0 (0) | 0 (0) | 3 (18.75) | ||
| NFATc1 expression | 0 | 10 (83.3) | 1 (7.7) | 0 (0) | p < 0.001 |
| 1 | 0 (0) | 2 (15.4) | 2 (12.5) | ||
| 2 | 0 (0) | 2 (15.4) | 4 (25.0) | ||
| 3 | 2 (16.7) | 8 (61.5) | 10 (62.5) | ||
IHC: Immunohistochemical
Protein expression in atherosclerotic coronary arteries.
Runx2 and Sox9 expression was absent in normal arteries (Fig. 2A, C). Nuclear staining of both factors in tunica intima of atherosclerotic arteries predominated in smooth muscle-like cells near calcified areas (Fig. 2B, D). Expression of both factors was also observed in smooth muscle cells of tunica media of atherosclerotic arteries with calcified lesions, while absent in tunica media of coronary arteries without calcification. Runx2 and Sox9 expression levels were significantly higher in advanced calcified lesions (p < 0.001) (Table 3). Moreover, Runx2 expression correlated with Sox9 (p = 0.008, r = 0.415) and RANK (p = 0.016, r = 0.380). However there was no association with cardiovascular risk factors.
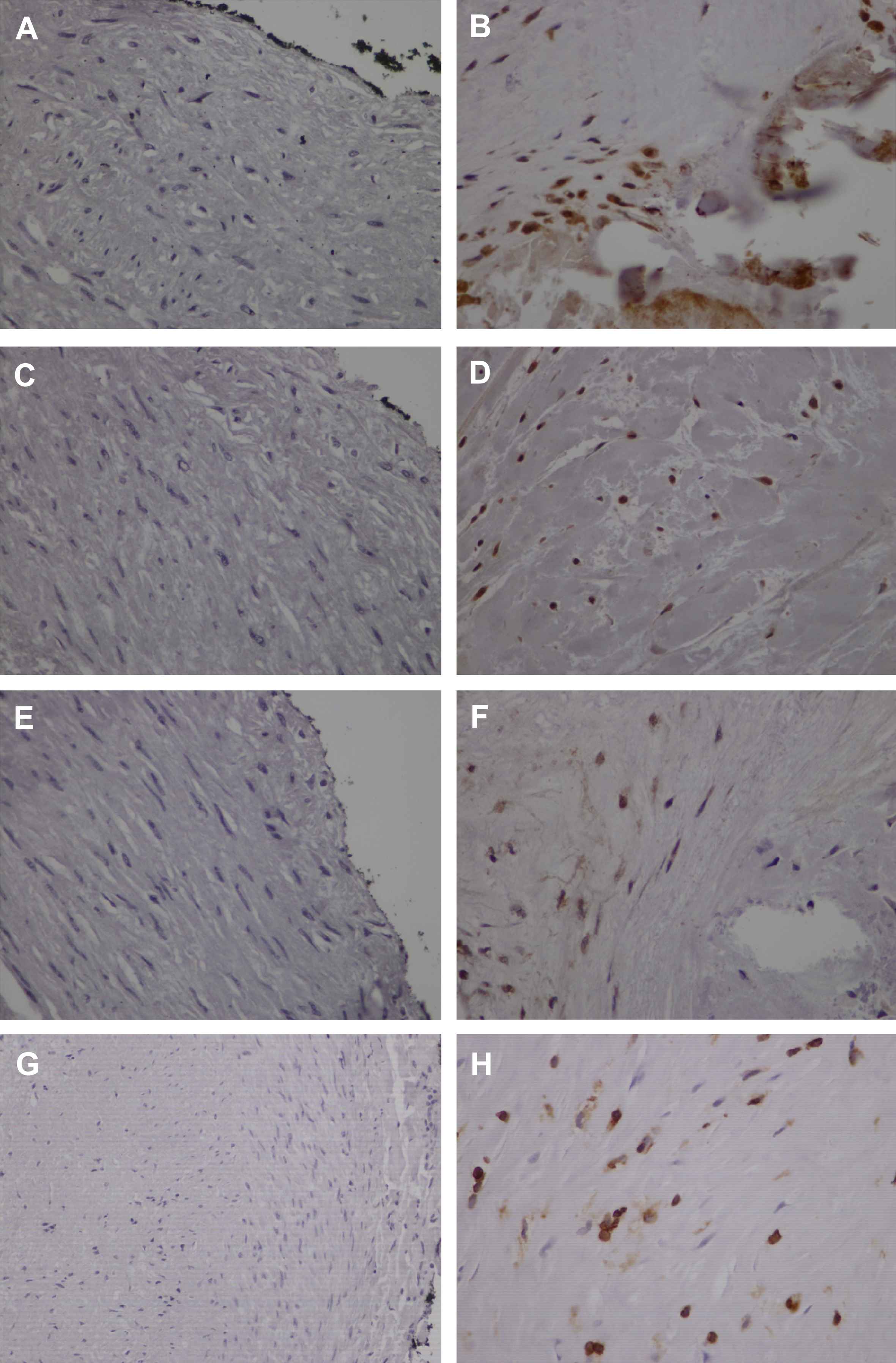
Bone-related factors Runx2, Sox9, Osterix and NFATc1 are overexpressed in human atherosclerotic coronary arteries. Negative staining for Runx2 (A), Sox9 (C), Osterix (E) and NFATc1 (G) in normal arteries. Representative cases of atherosclerotic arteries showing expression of Runx2 (B), Sox9 (D) and Osterix (F) in smooth muscle-like cells and intense immunopositivity of NFATc1 in smooth muscle-like cells and inflammatory cells (H). (×400).
Osx expression in normal arteries was absent (Fig. 2E). In contrast, nuclear localization of Osx was found in smooth muscle-like cells of tunica intima mainly in areas adjacent to calcification (Fig. 2F). Expression of Osx was significantly higher in stage 3 lesions (p < 0.001) (Table 3). No association with cardiovascular risk factors was observed. Osx expression correlated with Runx2 (p < 0.001, r = 0.616) and Sox9 (p = 0.04, r = 0.326).
Nuclear and cytoplasmic localization of NFATc1 was observed in inflammatory and smooth muscle-like cells of tunica intima of atherosclerotic coronary arteries while its expression was absent in control arteries (Fig. 2G, H). While there was no association with cardiovascular risk factors, NFATc1 showed significantly more intense nuclear expression in advanced lesions (p < 0.001) (Table 3). NFATc1 expression correlated with the expression of RANK (p = 0.025, r = 0.358), Runx2 (p < 0.001, r = 0.542), Sox9 (p = 0.001, r = 0.527) and Osx (p < 0.001, r = 0.544).
Discussion
Calcium deposition implies the presence of advanced atheromatous disease and is associated with increased cardiovascular mortality.36,37 A number of studies have shown intriguing parallels between arterial calcification and osteogenesis. The present study provides novel evidence that bone regulatory factors Osx and NFATc1 are implicated in coronary artery calcification.
We demonstrated that in contrast to normal arteries, Osx and NFATc1 are expressed in tunica intima of human atherosclerotic coronary arteries with levels of expression being significantly higher in advanced calcified lesions. Specifically, intense expression of Osx and NFATc1 was found in smooth muscle-like cells near to calcific deposits, while NFATc1 was also observed in inflammatory cells of tunica intima of atherosclerotic arteries. There was also a significant correlation between Osx and NFATc1 and both of them correlated with other bone regulatory factors. Osx is considered to be a crucial transcription factor in the terminal differentiation of osteoblasts and NFATc1 comprises not only a critical regulator of osteoclastogenesis but it is also implicated in the transcriptional control of osteoblast-related genes.20 Considering their significant role in bone differentiation, these findings suggest that Osx and NFATc1 expression may contribute to the orchestrated osteogenic processes involved in coronary artery calcification. Consistently with the present findings we have previously demonstrated increased expression of NFATc1 and Osx in calcified aortic valves.35
In addition to bone regulatory functions, NFATc1 is also known to regulate T cell maturation thus playing an important role in immune responses.23,24 Since inflammation is a major trigger for atherosclerosis and calcium deposition,37 it is not unlikely that NFATc1 may contribute to coronary artery calcification through both its bone-related and immunoregulatory functions.
In agreement with previous reports we also showed that OPG was expressed in smooth muscle cells of normal arteries while RANK and RANKL expression was undetectable.8,29,38–40 In atherosclerotic lesions RANKL and RANK expression was upregulated and notably expression of RANK was significantly higher in advanced calcified lesions, while OPG levels were invariable. Moreover, expression of RANK correlated with other bone regulatory factors such as NFATc1 and Runx2. However, a considerable number of subjects with atherosclerosis were receiving statin treatment. Statins possess pleiotropic effects other than their effects on the lipid metabolism.41 Recent works showed that statins modulate OPG mRNA and RANKL mRNA expression in mouse bone−cell cultures41 and reduce calcification in rat arteries and vascular smooth muscle cells (VSMCs).42 Therefore, lack of statistically significant difference in OPG and RANKL staining in atherosclerotic lesions in our material could be related to prior therapy with statin. Invariable expression of OPG and increased levels of RANKL have also been previously demonstrated in human atherosclerotic lesions and OPG −/− mice suggesting that elevated RANKL compared to OPG levels might favor vessel calcification.2,39,40 An increased RANKL/OPG ratio seems also consistent with the inflammatory nature of atherosclerosis since OPG often decreases while RANKL rises in several inflammatory settings.43,44 However, we need to interpret these results very carefully as other studies suggest a potentially deleterious effect of OPG on cardiovascular system. Increased serum OPG levels have been associated with presence and severity of coronary artery disease,45 peripheral arterial disease46 and heart failure due to left ventricular pressure overload.47 It still remains to be fully elucidated whether the observed increases in OPG levels are due to a compensatory defense mechanism or whether OPG has a causative role in pathophysiologic process.
We additionally provided evidence that in contrast to normal arteries, atherosclerotic coronary arteries showed expression of the master regulators of bone and chondrocytic differentiation, Runx2 and Sox9 respectively, mainly in calcified areas. Both Runx2 and Sox9 expression levels were significantly higher in advanced calcified lesions and correlated with other bone regulatory factors, findings supporting their implication in an active osteogenic programme that promotes mineralization of human atherosclerotic coronary arteries. Consistently, Runx2 and Sox9 expression has been also demonstrated in cultured human vascular cells as well as in calcified regions of aortic valves31 and human atherosclerotic vessels.6,30
In further support to the hypothesis that coronary artery mineralization resembles osteogenesis, several significant correlations regarding expression of bone regulatory factors in atherosclerotic lesions were demonstrated. In line with these findings, NFATc1 and Osx have been shown to cooperatively control osteoblastic bone formation25 and NFATc1 is also essential for RANK signalling during osteoclast differentiation.48 Osx induces the transcription of osteoblast-specific genes in response to Runx2 while Sox9 in known to function upstream of Runx2 in chondrocyte differentiation and bone formation.17–19 Moreover, correlation of RANK with osteoblast-specific factor Runx2 supports the hypothesis that in constrast to bone where RANKL/RANK activates bone absorption, in vasculature RANKL/RANK favors mineralization.2,39,40 Taken together, our results suggest that a highly regulated sequence of molecular events activates an osteogenic transcriptional programme in human coronary artery calcification.
Surprisingly, we did not observe any association between traditional cardiovascular risk factors and protein expression. It must be taken into account that a significant portion of study subjects were treated with statins and anti-hypertensive drugs. Several studies have shown that these agents interfere with progression of atherosclerosis.49,50
Present study had some limitations. Because the study population was small, we could not evaluate the inherent bias in the selection of patients for necropsy. Lack of statistical significance regarding expression of certain proteins such as OPG and RANKL among stages of atherosclerosis could be attributed to the study being underpowered.
In conclusion, our study provides further evidence that coronary artery calcification proceeds via active, highly regulated osteogenic processes in which the bone regulatory transcription factors NFATc1 and Osx seem to be critically implicated.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
Cite this article
TY - JOUR AU - Alexandros Alexopoulos AU - Stavros Peroukides AU - Vasiliki Bravou AU - John Varakis AU - Vlassios Pyrgakis AU - Helen Papadaki PY - 2011 DA - 2011/07/21 TI - Implication of bone regulatory factors in human coronary artery calcification JO - Artery Research SP - 101 EP - 108 VL - 5 IS - 3 SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2011.06.003 DO - 10.1016/j.artres.2011.06.003 ID - Alexopoulos2011 ER -
