The metabolic-microvascular dysregulation syndrome☆
Presented as the invited McDonald Lecture at the Artery 17 Meeting of the Association for Research into Arterial Structure and Physiology, Pisa, Italy, 12–14 October, 2017.
- DOI
- 10.1016/j.artres.2017.12.005How to use a DOI?
- Keywords
- Microcirculation; Microvascular function; Endothelium; Metabolism; Hyperglycaemia; Insulin resistance; Obesity
- Copyright
- © 2017 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
The McDonald Lecture honours Donald Arthur McDonald (1917-1973), a British physiologist who established the modern approach to the study of arterial haemodynamics over a 20-year period from 1953-1973. His work established the logic of using Fourier analysis to break down pressure and flow waves, and developed the general concept of vascular impedance.1 His classic book on blood flow in arteries was published in 1960,2 and has remained a basic treatise in this field for more than 50 years.3 Linking with engineers and with physicians, he influenced many young physicians and physiologists. He directed work into the clinical sphere while continuing in basic physiology and haemodynamics.1 Donald McDonald thus is an intellectual godfather of the ARTERY society, and I am honoured to present the 2017 McDonald Lecture.
ARTERY’s goal is to promote the advancement of knowledge and dissemination of information concerning the pathophysiology, pharmacology, epidemiology, detection, investigation and treatment of arterial structure and function. Thus its goal is to further the understanding of human diseases from the point of view of large artery structure and function. As a clinical scientist, I submit that understanding human disease is impossible without crossing borders, notably that from large arteries to the microcirculation. Indeed, the society’s journal, Artery Research, publishes papers not only in the classic domain of arterial structure and function and its interaction with various organs such as the heart, kidney and brain, but has on occasion published papers that focus entirely on the microcirculation.4,5
The microcirculation is widely taken to encompass vessels <150 μm in diameter. It therefore includes arterioles, capillaries, and venules. A definition based on arterial vessel physiology rather than diameter or structure has also been proposed, depending on the response of the isolated vessel to increased internal pressure. By this definition, all vessels that respond to increasing pressure with a myogenic reduction in lumen diameter are considered part of the microcirculation, including the smallest arteries and arterioles in addition to capillaries and venules. A primary function of the microcirculation is metabolic, to optimize the delivery of nutrients and removal of waste products in response to variations in demand. A second important function is to avoid large fluctuations in hydrostatic pressure at the level of the capillaries that otherwise would impair capillary exchange. Finally, it is at the level of the microcirculation that a substantial proportion of the drop in hydrostatic pressure occurs. The microcirculation is, therefore, extremely important in determining the overall peripheral resistance. In normal conditions, systemic, regional, and local metabolic and myogenic autoregulatory mechanisms ensure adequate progress of these microcirculatory functions. In pathological conditions, however, the loss of such mechanisms results in the development of microvascular dysfunction.6
At the ARTERY14 meeting, in 2014, Alun Hughes elegantly considered the design of the circulation (large and small vessels) from the point of view of optimality and cost minimization.7 In this McDonald Lecture, I shall take this one step further and develop the concept that microvascular and metabolic physiology are inextricably linked. Indeed, I shall postulate that dysfunction of the one causes dysfunction of the other, justifying the concept of a ‘Metabolic-Microvascular Dysregulation Syndrome’.
Microvascular consequences of metabolic dysregulation
Diabetic retinopathy is the classic example of the link between metabolism and microvessels. In diabetic retinopathy, metabolic dysregulation (hyperglycaemia) causes many microvascular changes, such as microaneurysms, haemorrhages, and hard and soft exudates.8 There is convincing evidence that the link is causal; thus, reduction of hyperglycaemia reduces onset and progression of retinopathy.9,10
Diabetic nephropathy is a second example. Morphologically, diabetic nephropathy is less exclusively microvascular than is diabetic retinopathy, and is characterized by arteriolar hyalinosis but most typically by glomerular basement membrane thickening and mesangial expansion.11 Nevertheless, microvascular endothelial dysfunction has been shown to be a core feature of diabetic nephropathy. Functionally, there is first an increase in glomerular filtration rate (hyperfiltration), followed by a steady decrease over time. In parallel, urinary albumin excretion increases from normal (<30 mg/24h) to microalbuminuria (30-300 mg/24h) and macroalbuminuria (>300 mg/24h).12 It is in the urinary leakage of albumin that microvascular endothelial dysfunction is especially important. A key observation, in the 1980s and 1990s, was that in type 2 diabetes, type 1 diabetes and in the general population even a slight increase in urinary albumin excretion (microalbuminuria) was associated with a large increase in risk of cardiovascular events.13–15 This association has in many studies been shown to be independent of conventional risk factors and persists over many years.16 From renal physiology it follows that excess albuminuria must be explained by excess permeation across the glomerular capillary wall (itself dependent on glomerular pressure, permeability and surface area) and/or impaired renal tubular reabsorption.17 Cell types involved include podocytes, microvascular (arteriolar and glomerular) endothelial cells, mesangial cells and tubular cells. Of these, endothelial cells, if dysfunctional not only in the kidney but also elsewhere, could potentially explain the link between microalbuminuria and cardiovascular disease (i.e., atherothrombosis).
In late 1980s, we set out to investigate this concept with then available markers of microvascular endothelial dysfunction, such as plasma levels of von Willebrand factor and, later, of adhesion molecules (sVCAM-1, sICAM-1, sE-selectin). We found that levels of such biomarkers were strongly associated with onset and progression of microalbuminuria in type 1 and type 2 diabetes as well as in the general population,18–20 as reviewed elsewhere.21 It took many years to develop more direct methods to investigate microvascular endothelial function at the population level.22 Studies using such methods further supported the concept of microalbuminuria as a marker of microvascular endothelial dysfunction. In the Maastricht Study,23 albuminuria was associated with capillary density (assessed in skin),24 heat-induced microvascular dilation (also assessed in skin), and flicker-light-induced arteriolar dilation (assessed in the retina) (Martens et al, unpublished observations).
Taken together, these findings establish that relatively severe hyperglycaemia, as in diabetes, can cause microvascular disease (retinopathy and albuminuria). We recently showed that less severe hyperglycaemia (prediabetes) is also associated with microvascular dysfunction. The association between glycaemia and microvascular dysfunction in fact appears not to have a threshold and can be demonstrated in skin25,26, retina25,26 and brain, in the latter as white matter hyperintensities (Van Agtmaal et al, unpublished observations), which are thought to represent cerebral small vessel disease.
Microvascular dysfunction demonstrated with such methods is clinically relevant. For example, biomarkers of microvascular dysfunction are associated with depression both cross-sectionally and longitudinally;27,28 microalbuminuria is associated not only with myocardial infarction and stroke but also with depression27,28 and impairment of cognitive function;29 and white matter hyperintensities predict stroke, dementia, depression and mortality (Rensma et al, unpublished observations).
Taken together, there is a continuous, presumably causal, association between glycaemia and microvascular dysfunction, which does not have a clear threshold, and which predisposes to clinical disease.
Metabolic consequences of microvascular dysregulation
Observations over the past 25 years have convincingly shown that microvascular dysregulation impairs normal metabolism.6 Insulin is an endothelium-dependent vasodilator.30 In animal models, insulin can recruit previously underperfused nutritive capillary networks in skeletal muscle.31–33 This action of insulin on the microcirculation enhances insulin-mediated glucose disposal and indeed is necessary for normal insulin sensitivity; conversely, microvascular endothelial dysfunction impairs insulin-mediated glucose disposal, i.e. causes insulin resistance.7,33 Using skin as a model, we were the first to show that insulin has similar microvascular actions in humans34–36 and that insulin-induced microvascular recruitment contributes to glucose disposal independently of visceral, subcutaneous and intrahepatic fat.37 Interestingly, an animal model in which insulin signal transduction is selectively impaired in endothelial cells (and normal everywhere else) shows not only impaired insulin-induced microvascular dilation and whole-body glucose disposal,38 but also impaired insulin secretion,39 suggesting that normal endothelial function is needed for normal glucose-induced insulin secretion. This has yet to be demonstrated in humans.
The studies reviewed above show that normal microvascular endothelial function is necessary both for normal insulin-mediated glucose disposal and for glucose-induced insulin secretion. It logically follows that impaired microvascular endothelial function should predispose to the development of type 2 diabetes. We have found that indeed to be the case, regardless of how microvascular dysfunction was measured.40 Thus, the association between hyperglycaemia and microvascular endothelial dysfunction is bidirectional. This constitutes the core of the Metabolic-Microvascular Dysregulation Syndrome.
Obesity as a driver of the metabolic-microvascular dysregulation syndrome (Fig. 1)
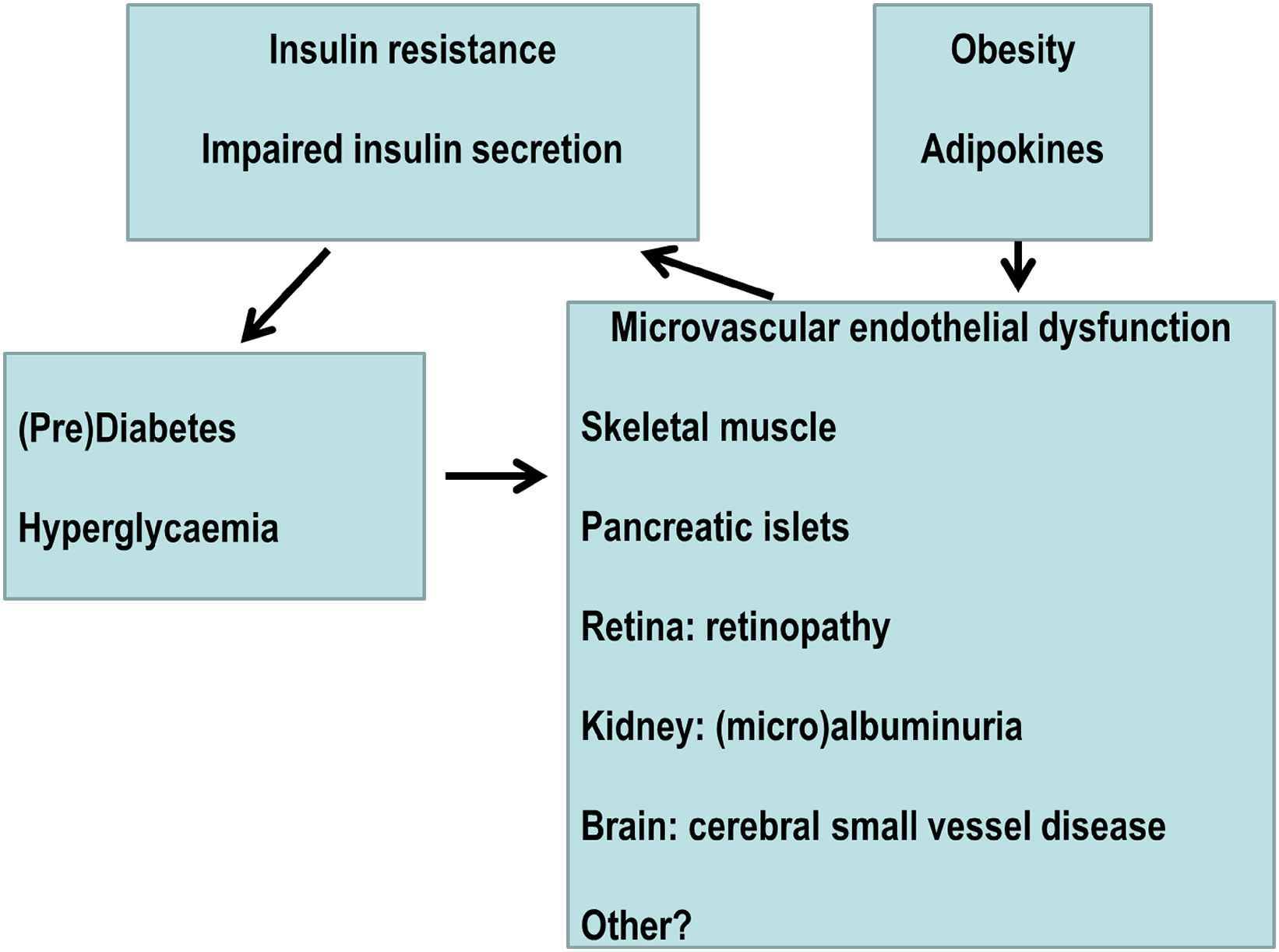
Overview of the Metabolic-Microvascular Dysregulation Syndrome.
Obesity is associated with microvascular dysfunction, including impaired insulin-induced microvascular dilation and recruitment.37,41–43 Normal insulin action in endothelial cells is to increase synthesis of nitric oxide through the PI3 kinase pathway more than endothelin synthesis through the ERK pathway, with vasodilation as the net result.44–46 Obesity shifts this balance towards less vasodilation or even vasoconstriction through adverse changes in adipokines such as adiponectin, free fatty acids and tumour necrosis factor-α, which impair insulin signal transduction in endothelial cells to impair nitric oxide synthesis and/or enhance endothelin production.47–53 For example, in lean humans, an acute increase in FFA levels impairs microvascular recruitment in response to reactive hyperaemia and insulin; conversely, in obese humans, an overnight decrease in FFA levels induced by acipimox increases microvascular recruitment in response to reactive hyperaemia and insulin.54 These adipokines may derive from not only visceral fat but also from subcutaneous (truncal) and perivascular fat.37,53,55
Microvascular dysfunction in obesity appears reversible. In abdominally obese men, diet-induced weight loss (∼10 kg in eight weeks), as compared to a control group receiving a weight-maintenance diet, improved whole-body glucose disposal in part through improvement of insulin-induced microvascular recruitment in skeletal muscle.37 Abdominal obesity was also associated with a lower retinal arteriole-to-venule ratio, which normalised after weight loss.56 In summary, obesity is associated with widespread microvascular dysfunction, which is at least partly reversible on weight loss.37,56
Perspectives
Obesity is a major factor in setting up the syndrome, but other factors are also likely to play a role. I shall briefly discuss three of these. Firstly, and fascinatingly, the hypothesis has been advanced that microvascular dysfunction of adipose tissue is a primary cause of adipose tissue dysfunction resulting in adverse changes in adipokines.57 These findings57 raise the possibility that microvascular dysfunction is at the very core of the Metabolic-Microvascular Dysregulation Syndrome. Secondly, early life exposures, both antenatal and postnatal, are likely to be important. For example, in otherwise healthy individuals born at term, low birth weight is associated with impaired microvascular function, both in adults58 and in prepubertal children.59 In healthy newborns, smaller size at birth and maternal hypertension were associated with impaired microvascular endothelial function;60 and rapid growth in the first month was inversely associated with microvascular endothelial function at six months of age.61 This observation suggests that rapid growth can be detrimental to microvascular endothelial function. In this respect, the early postnatal period may be a critical period and provide a window of opportunity for prevention of microvascular endothelial dysfunction, for example through nutritional means.61 Finally, what of arterial stiffening? Stiffening of large arteries impairs their cushioning function and increases pressure and flow pulsatility, which transmits distally and can damage the microcirculation.62,63 Indeed, previous studies have shown an association between greater arterial stiffness and markers of microvascular dysfunction in the brain (cerebral small vessel lesions),64 eye (retinal arteriolar narrowing),65 and kidney (microalbuminuria).66,67 These organs are especially vulnerable to the detrimental effects of increased pressure and flow pulsatility, as their microvasculature is characterized by low impedance, allowing the pulsatile load to penetrate deeply into their microvascular beds.62,63 In contrast, there is no strong evidence that other organs are similarly affected. For example, we have not found significant associations between aortic or carotid stiffness and plasma biomarkers of microvascular endothelial dysfunction68,69 or an array of estimates of skin microvascular function.70 Thus, the microcirculation of most organs may be able to protect itself against the detrimental effects of increased arterial stiffness and pressure and flow pulsatility through relatively high microvascular impedance as a result of effective autoregulation and/or vascular remodelling. This would dissipate most of the increased pulsatile energy by arteries and large arterioles proximal to capillary beds and thus limit penetration of the pulsatile load. In sum, large artery stiffening is unquestionably important for microvascular function in susceptible organs such as the brain, the eye and the kidney, but there is no clear evidence that this is a generalized phenomenon. It is therefore not clear that arterial stiffening in and of itself is sufficient to cause the Metabolic-Microvascular Dysregulation Syndrome. It should be kept in mind, however, that associations between arterial stiffening and microcirculatory function in organs that are probably essential for setting up the syndrome, such skeletal muscle, pancreas and adipose tissue, have yet to be examined.
Conclusions
Microvascular and metabolic physiology are inextricably linked. I here propose that dysfunction of the one causes dysfunction of the other, justifying the concept of a ‘Metabolic-Microvascular Dysregulation Syndrome’. For example, metabolic dysregulation (hyperglycaemia) causes microvascular dysfunction, diabetic retinopathy and diabetic nephropathy. Conversely, microvascular dysregulation impairs insulin-mediated glucose disposal, i.e. causes insulin resistance, impairs insulin secretion, and is associated with onset of type 2 diabetes in prospective studies. Obesity is a key driver of the Metabolic-Microvascular Dysregulation Syndrome, as it impairs insulin signal transduction in endothelial cells through adverse changes in adipokines such as adiponectin, free fatty acids and tumour necrosis factor-α. Microvascular dysfunction in obesity appears reversible by diet-induced weight loss. Next to obesity, other factors are also likely to play a role. Examples are microvascular dysfunction of adipose tissue as a primary cause of adipose tissue dysfunction; early life exposures, both antenatal and postnatal; and large artery stiffening. Large artery stiffening is unquestionably important for microvascular function in susceptible organs such as the brain, the eye and the kidney but whether it can cause microvascular dysfunction in metabolically crucial tissues such as skeletal muscle, pancreas and adipose tissue has not been studied. It is therefore not clear that arterial stiffening in and of itself is sufficient to cause the Metabolic-Microvascular Dysregulation Syndrome.
References
Cite this article
TY - JOUR AU - Coen D.A. Stehouwer PY - 2018 DA - 2018/01/10 TI - The metabolic-microvascular dysregulation syndrome☆ JO - Artery Research SP - 78 EP - 83 VL - 21 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2017.12.005 DO - 10.1016/j.artres.2017.12.005 ID - Stehouwer2018 ER -
