A comparative study of vasa vasorum density among coronary arteries
- DOI
- 10.1016/j.artres.2018.04.002How to use a DOI?
- Keywords
- Adventitial vasa vasorum; Atherosclerosis predisposition; Histological staining technique
- Abstract
Background: Coronary arteries exhibit significantly higher adventitial Vasa vasorum (VV) densities which may partly explain their higher atherosclerosis susceptibility. A variability in the atherosclerosis predispositions exists between individual coronary artery branches but few studies exist that compare their VV densities. This study aims to assess for VV density differences between individual coronary artery branches and also compare them with other similar sized peripheral arteries.
Methods: Cadaveric arterial specimens, 15 each, from right coronary [RCA], left anterior descending [LAD], left circumflex [LCX] radial [RA] and fibular arteries [FA] were collected, processed and stained. Images were taken using digital Motic camera enabled microscope and Motic Image plus 2.0 ML software. VV were visually identified and counted. Open source software Image J was used to measure the adventitial surface area. VV number per unit area was expressed as density.
Results: Mean adventitial VV density (*10−5/μm2) was significantly higher in the RCA (2.14 SD 0.92) compared to the LAD (1.2 SD 0.72) RA (1.23 SD 0.41) and FA (0.77 SD 0.84). VV density in LAD was comparable to LCX (1.88 SD 0.83), RA and FA
Conclusion: LAD showed the least VV density among coronary arteries, even though most studies show this artery to have the highest atherosclerosis incidence. Interactions between high and low VV density areas within an artery rather than net VV density may be a more significant determining factor for atherosclerosis predisposition. Structural and molecular characteristics of the vessel wall, as well as that of VV, may also be contributing factors.
- Copyright
- © 2018 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Atherosclerosis, characterised by sub intimal accumulation of oxidised LDL and inflammatory cells in the walls of arteries, does not uniformly afflict all arteries.1 The coronary, popliteal, internal carotid and basilar arteries are known to be more prone for atherosclerosis compared to the radial and internal mammary arteries.2,3
Current studies have focused on the role of Vasa vasorum (VV) in the pathogenesis of atherosclerosis to help explain these differential susceptibilities.
The primary initiator of atherosclerosis is known to be endothelial dysfunction triggered by various risk factors such as smoking, diabetes mellitus, hypertension, and dyslipidemia. VV are now thought to be the primary source of response to this type of injury.4 According to the outside-in model of atherosclerosis, the inflammation associated with the disease is thought to originate in the arterial adventitia where the VV serve as conduits for cellular migration. Animal studies have shown VV to be reservoirs of multipotent progenitor cells, giving rise to cellular elements involved in plaque formation and progression.5 Inflammatory cell accumulation has been observed in the adventitia overlying the site of plaque in atherosclerotic arteries.6
Some studies suggest VV dysfunction itself triggers plaque formation.7 Intimal hyperplastic lesions appear upon removal of carotid artery adventitia in rabbits fed on a high cholesterol diet. A resultant arterial wall hypoxia is thought to be responsible for this observation.8
A function attributed to VV is solute clearance from within the arterial wall. A transmural solute flux is suggested to normally occur between the arterial lumen and the adventitial VV, favored by a transmural pressure gradient. Reduced solutes clearance due to dysfunctional VV can cause solutes to accumulate and deposit in the arterial wall, leading to plaque formation7. Studies done on rat coronary arteries have shown atherosclerotic plaques formation to occur following obstruction of the venous VV.9
Vasa vasorum are thought to function as conduits for the transfer of mediators from the perivascular fat to the inner layers of the vessel wall and abnormalities in this may also play a role in atherosclerosis.10
Many studies have found a positive co-relationship between predisposition of certain arteries to atherosclerosis and their VV densities.11–13
Studies that have used Micro-CT to determine VV density in porcine arteries have shown densities to be highest in the coronary arteries, intermediate in the renal and carotid arteries and lowest in the femoral arteries.11 Similar studies using three-dimensional microscopic computed tomography on human arterial specimens obtained at necropsy have also shown VV density to be three-times higher in coronary compared to the renal and femoral arteries.12 VV densities have been found to be significantly higher in the coronaries compared to the internal thoracic artery, which is rarely affected by atherosclerosis.13
Most studies that have assessed VV densities do not specify which coronary artery branches were analysed and whether differences existed in VV densities between the individual coronary arteries.11–13
Since it is known that coronary artery branches are all not uniformly susceptible to atherosclerosis, we proposed to look for VV density differences between individual branches to see if they correlated with the artery’s disease susceptibility.14 We also compared the VV densities between the coronary artery branches and other similar sized peripheral arteries.
In the clinical setting, measuring the density of VV (in vivo) is considered an emerging technique to detect early development of atherosclerosis and vulnerability of plaques.15 Intravascular ultrasound is being used to detect the hypoechoic VV.16 Other methods such as Optical Coherence Tomography where VV show up as a signal-poor tubuloluminal structure are also being used to detect plaque vulnerability, by measuring VV density.17
Two-dimensional histological sections of the coronary arteries have been used in previous studies to ascertain the number of VV in the adventitia, by manual counting, as they are visualised under the microscope. The area of the adventitia is measured, and the density of VV is calculated by dividing the number of VV by the area of adventitia.18 The In-vivo techniques of assessing VV density have been compared with the histological staining techniques and VV densities measured have been found to be comparable.15
Aims & objectives
To find out if significant differences exist in the adventitial VV density between individual coronary arteries and to compare these VV densities with those observed in other similar sized peripheral arteries.
Methods
After obtaining clearance from the Institutional Ethics Committee (IEC No: 338/2017), cadaveric arterial specimens, 15 of each, of unknown sex and age were collected from the Department of Anatomy, Kasturba Medical College, Manipal.
Sample collection
Small segments of the right coronary artery [RCA], left anterior descending artery [LAD] and the left circumflex artery [LCX] were taken at its origin. The radial artery [RA] segments were collected from the anatomical snuff box and the fibular artery [FA] from its origin as a branch of the posterior tibial artery.
The arteries were identified and cleaned with blunt forceps avoiding injury to the vessel wall. Arterial segments were cut, using fresh scalpel blades for each section. The sections were taken under running tap water to remove the excess fat and connective tissue.
For the purpose of standardization, all sections were taken at the origin of the respective arteries.
Preparation of sections
The tissue samples collected were left under running tap water overnight to wash off the formalin fluid. The process of paraffin block preparation was done first by dehydration of the specimens in ascending grades of alcohol-a) 50% alcohol for 24 h, b) 70% alcohol for 24 h c) 90% alcohol for 12 h d) absolute alcohol for 12 h
The samples were then cleared in xylene till the tissues became transparent. They were infiltrated in paraffin wax by placing them sequentially in four different containers, each for 30 min. Paraffin blocks were prepared using L pieces with fresh molten paraffin. Paraffin sections of 5-micron thickness were cut, and the sections were allowed to float in a water bath that was maintained at a temperature 60 °C. The sections were then mounted on gelatinized slides after which, they were placed on a slide warmer.
Staining of the sections
The slides were stained with Hematoxylin & Eosin (H &E) stains. Before staining, deparaffinization of the sections was done in xylene (for 5 min).This was followed by hydration of the sections, first with graded alcohol (3 min each with 100%, 90%, 70% and 50% alcohol) and then with distilled water (for 5 min). The slides were then stained with Hematoxylin (for 5 min) after which the slides were placed under running tap water for 10 min. Following this, the slides were kept in Eosin solution (for 1 min), then washed in 90% and 100% alcohol and finally cleared in xylene. The stained sections were mounted with DPX (Distyrene, plasticizer, xylene) and observed for the following features: VV and layers of the vessel wall.
Identification
The tunica media in medium-sized arteries predominantly comprise of smooth muscle and was identified as the layer spanning the internal and the external elastic lamina.
The layer outside the external elastic lamina was identified as the tunica adventitia comprising mainly of longitudinally arranged collagen fibres and elastic connective tissue fibres. VV were identified here as endothelium-lined small vessels (See Figs. 1–3).
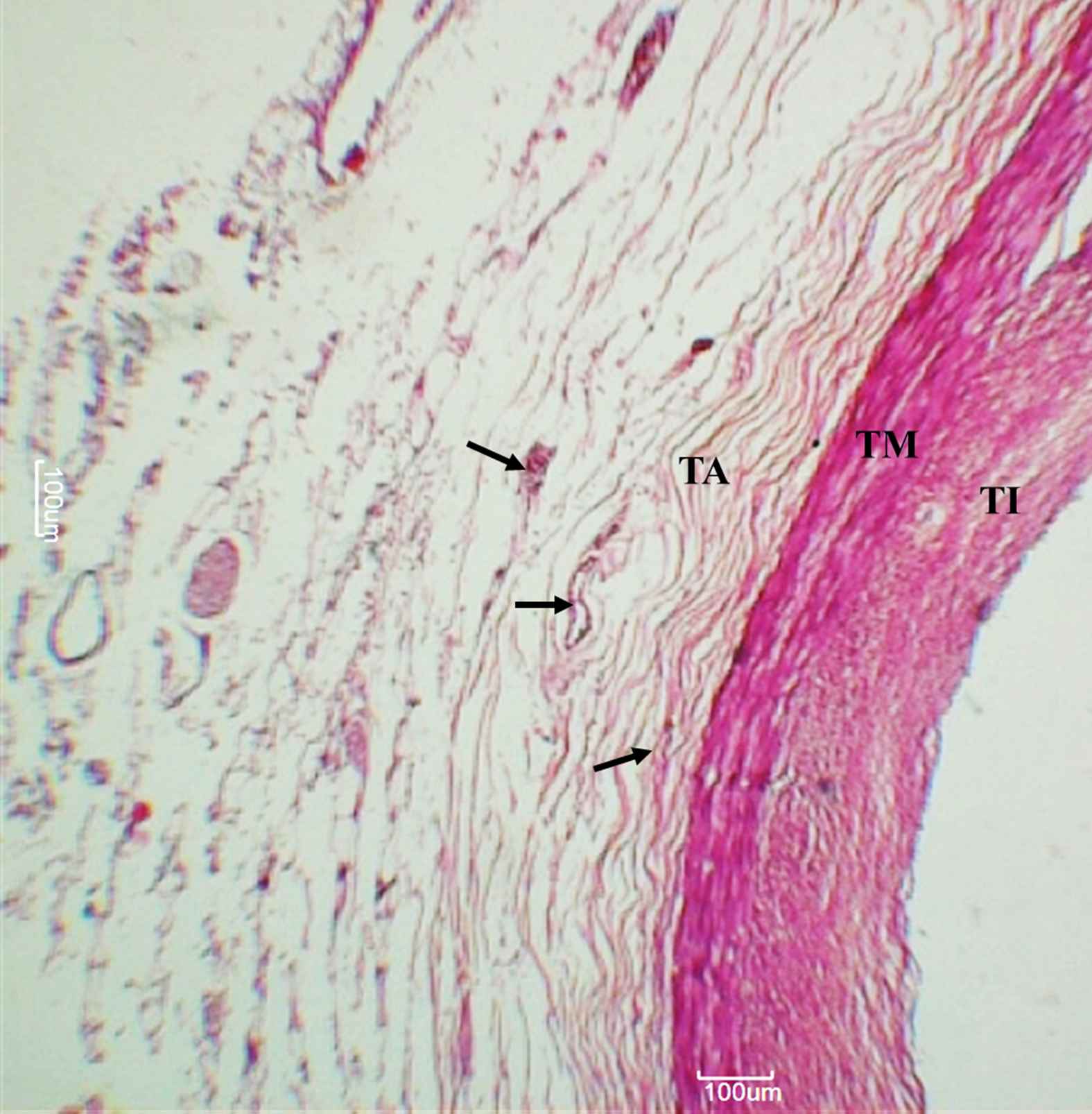
Right coronary artery. Arteries, as observed under 50× magnification. Arrowheads indicate adventitial vasa vasorum. TI – Tunica intima, TM – Tunica media, TA – Tunica adventitia.
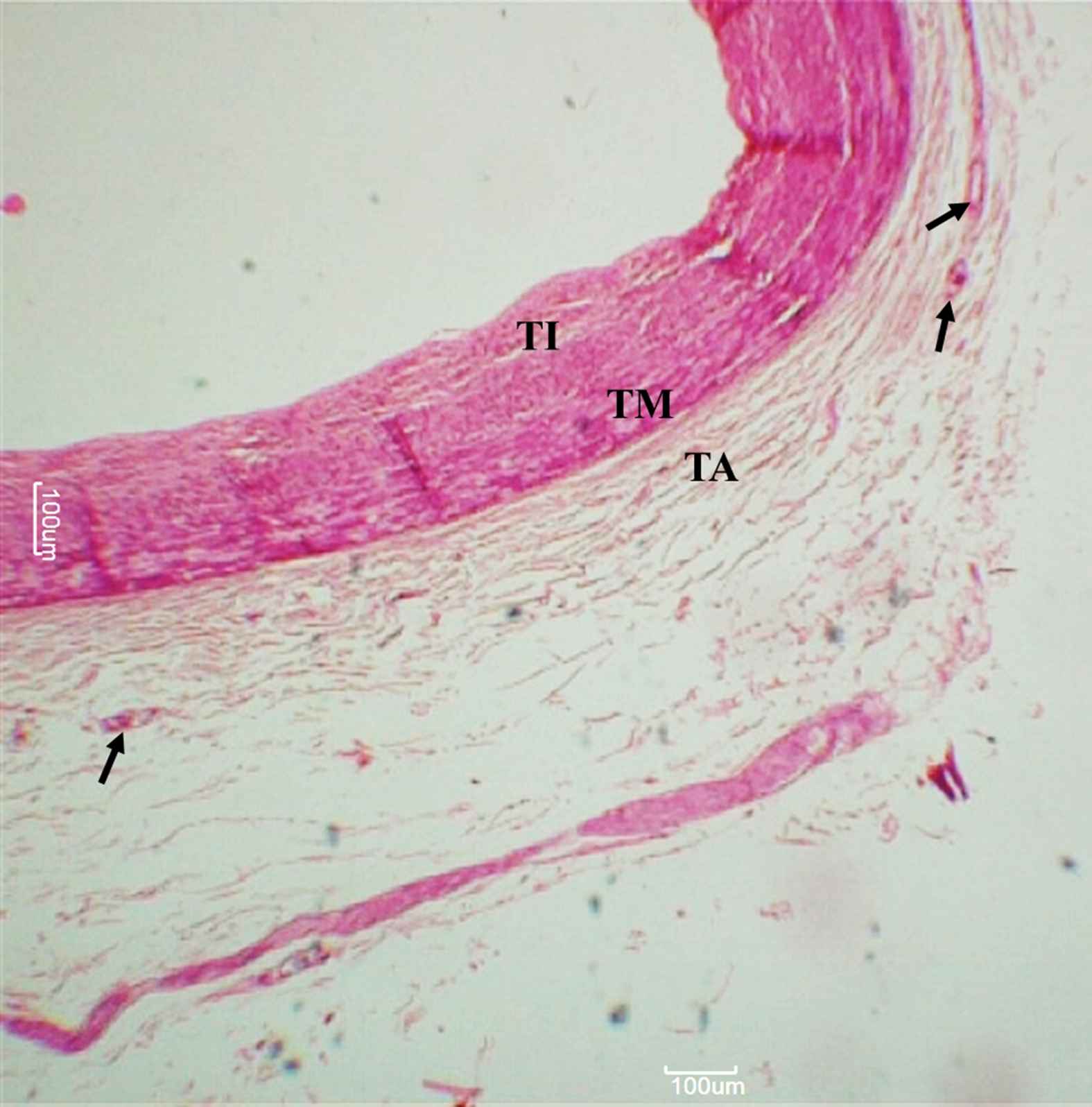
Left circumflex artery. Arteries, as observed under 50× magnification. Arrowheads indicate adventitial vasa vasorum. TI – Tunica intima, TM – Tunica media, TA – Tunica adventitia.
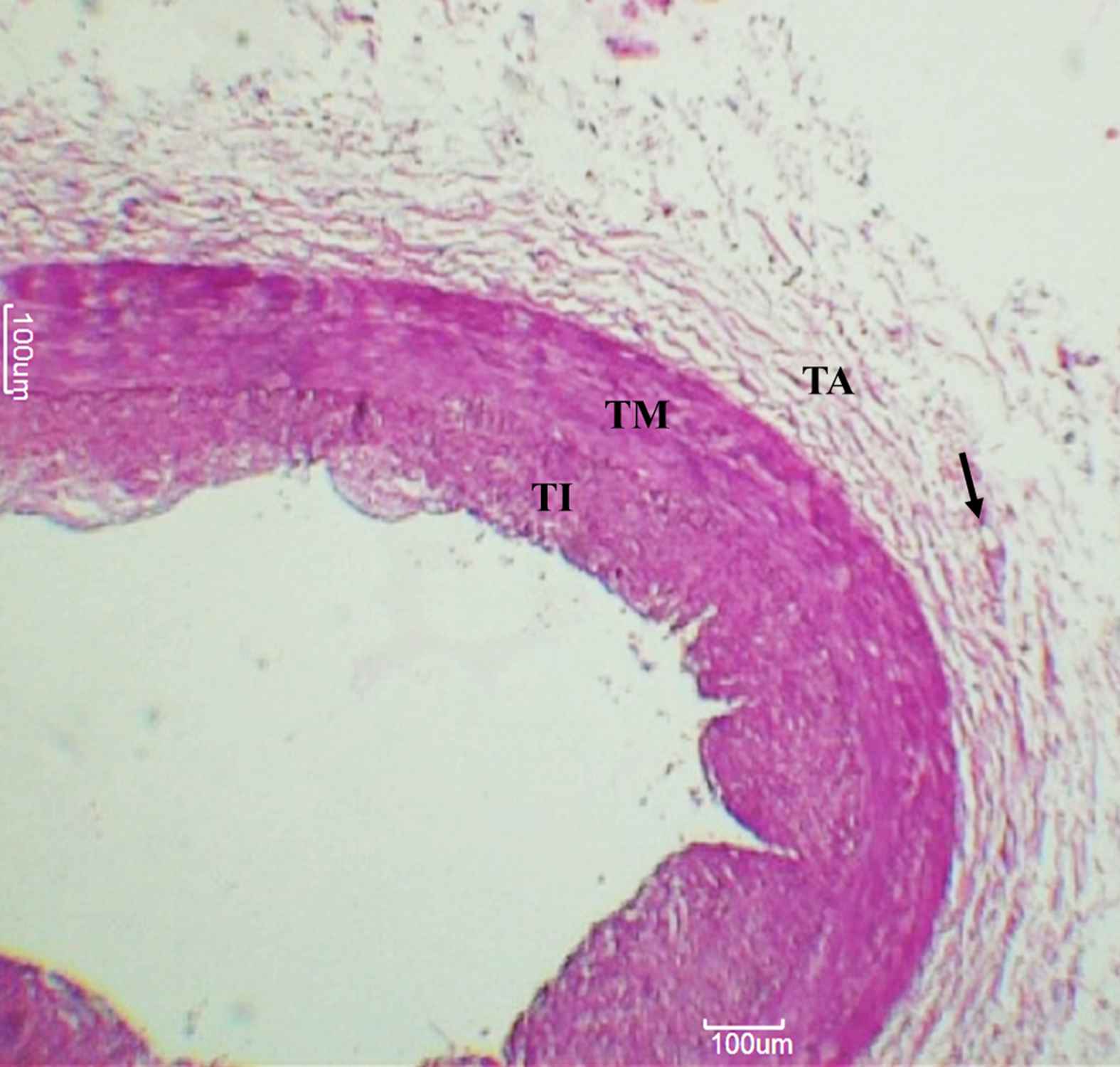
Left anterior descending artery. Arteries, as observed under 50× magnification. Arrowheads indicate adventitial vasa vasorum. TI – Tunica intima, TM – Tunica media, TA – Tunica adventitia.
All slide sections were named after the arteries and serially numbered.
Each slide contained on an average 5 histological sections obtained from one specimen of a particular artery type.In each slide, the section in which the artery layers and VV were most distinctly visible was chosen for counting.
Measurement and analysis
Images were taken with the help of digital Motic camera enabled microscope and the Motic Image plus 2.0 ML software. Initially, the Microscope and the software were calibrated at 50× magnification (5× scanning objective was used), and the software was set to record a scale bar of 100 um with every image.
The area measurements were done with the help of the open source software Image J. After opening the images in Image J, calibration was done using the Set Scale option. Adjacent non-overlapping areas in the tunica adventitia in each section were selected and area measurements were done using the freehand selection Tool.19 On an average, four non-overlapping areas were chosen per specimen in order to cover the entire circumference of the artery. The VV number in all the selected areas of the specimen were visually calculated, and the number per unit area was later expressed as density. The mean VV density for a particular artery was calculated by taking the average of the densities obtained in all the specimens of the artery type.
Data was compiled and analyzed in Statistical Package for the Social Sciences (SPSS) version 15.0. ANOVA was used to compare the mean densities of the different arteries. A p value of less than 0.05 was taken to be statistically significant.
Results
The density of adventitial VV was highest in the RCA at 2.14 *10−5/μm2, followed by the LCX at 1.88 *10−5/μm2 and the LAD at 1.20 * 10-5/μm2.Among the peripheral arteries, the RA had a VV density of 1.23 *10−5/μm2 and the FA had a VV density of 0.77 *10−5/μm2 (Table 1).
| Mean (*10−5) | Std. Deviation (*10−5) | Std. Error of mean (*10−5) | |
|---|---|---|---|
| RCA | 2.14 | 0.92 | 0.24 |
| LCX | 1.88 | 0.83 | 0.21 |
| LAD | 1.20 | 0.72 | 0.18 |
| RA | 1.23 | 0.41 | 0.1 |
| FA | 0.77 | 0.84 | 0.22 |
VV density of Right coronary and Left circumflex is higher than that of the left anterior descending, Radial and Fibular arteries.
Mean, standard deviation (*10−5) and standard error of mean (*10−5) for adventitial vasa vasorum densities (VV count/μm2 of adventitia) in different artery groups.
ANOVA analysis revealed a significant difference between densities of VV in various artery groups. [F (4, 60) = 6.728, p < 0.001]. Between the coronary arteries, Post hoc Bonferroni test revealed that the VV density of RCA was significantly higher than that of LAD (p = 0.03) but not LCX. The difference in VV density between LAD and LCX was not statistically significant.
Between the coronary and peripheral arteries, VV density of RCA was significantly higher than that of RA (p = 0.018) and FA (p = 0.001). The VV density of LCX was significantly different only from the VV density of FA (p = 0.008).VV density of LAD was comparable to that of the peripheral arteries with no significant difference observed.
Discussion
Differences in the VV density exist among different vascular beds and these differences are thought to make arteries respond differently to the same exposure to atherosclerosis risk factors. This factor is used to explain why the coronary arteries which have a higher VV density also are more susceptible to the process of atherosclerosis.11
Studies done to study atheroscleosis incidence have shown the Left coronary artery and its branches to be affected by atheroscleosis more often than the right coronary artery.14 Necropsy studies and studies done using cardiac computed tomography angiography (CCTA) have shown that incidence of plaque and calcium deposits is highest in the left anterior descending artery followed by the right coronary artery and least in the circumflex branch.20
Considering that studies suggest high VV densities are favourable for plaque formation, we had proposed to find the highest VV density in the left anterior descending coronary artery.11,12
However contrary to that, in our study we found a significantly higher density of VV in Right coronary artery in comparison to the LAD and LCX which does not positively correlate with the incidence of atherosclerosis observed in these arteries.
In addition to that, the VV density of the LAD was not significantly different from the peripheral arteries which normally exhibit much lower atherosclerosis incidence compared to the coronaries.
This apparent contradiction could be explained using studies done on porcine right coronary arteries. In the study, in which animals fed on a high cholesterol diet, segments of low VV density, as assessed with micro CT, showed greater evidence of hypoxia, micro-inflammation and oxidative damage.21 Within the LAD, the myocardial side of the artery is known to be more prone to atherosclerosis. However, on anterograde perfusion of porcine LAD with high intraluminal pressure, lower venous VV density was observed on the myocardial side of the arteries compared to the epicardial side.22
These findings suggest the possibility that high VV densities may be atheroprotective by preventing hypoxia and oxidative damage as well as by enhancing solute clearance from the arterial wall.9,21 This may help partly explain why a high VV density was observed in the less frequently affected RCA in our study.
It may also be possible that the net VV density of the arteries is less of a determinant for atherosclerosis predisposition. A regional heterogeneity in VV density may exist within arterial segments, where areas of low VV density serve as sites of plaque initiation.21 Plaque progression may on the other hand be favoured by adjacent areas of high VV densities. Plaque progression, as well as plaque vulnerability to rupture, is determined principally by the degree of neovascularisation. This neovascularisation occurs through branching of new VV from the existing adventitial VV, in response to hypoxia and release of angiogenic mediators like VEGF.1,23 A high existing density of adventitial VV within the arterial wall may therefore support more neovascularization and favour plaque progression. Thus,while the initiation of atherosclerosis may be favoured by low VV densities, plaque progression into the advanced stage may be supported by higher VV densities. Neovascularization in the plaque serves to enhance VV density, which in turn could serve as a marker of atherosclerosis severity.
Causes other than VV density may also be considered as factors that determine atherosclerosis predisposition of the coronary arteries. Regional differences in blood flow characteristics may cause endothelial cells to respond differently to risk factors. Endothelial shear within normal ranges is thought to be atheroprotective.20 Shear stress is known to inhibit smooth muscle cell migration by causing an increase in Platelet-derived growth factor-A.24 Shear stress also maintains endothelial function by preventing apoptosis, and low shear stress is known to increase oxidative stress and decreases Nitric oxide synthase activity.25,26
In contrast to the right coronary which exhibits a more uniform unidirectional blood flow, the left artery shows lesser systolic flow which decreases endothelial shear. The left coronary artery is also exposed to higher torsion and shows more branching, all of which might increase its atherosclerosis susceptibility.14
Studies done on porcine arteries have shown that within the LAD, although no significant differences exist in the VV densities between segments, the proximal third which is more prone for atherosclerotic plaque rupture also showed a higher absolute VV count in comparison to the middle and distal third of the artery.27 Also, the vascular area fraction (∑vasa vasorum areas/vessel wall area; %) and endothelial surface fraction (∑vasa vasorum endothelial surface areas/vessel wall volume; mm2/mm3) were significantly higher in the proximal LAD in control animals.27 Future studies could look for differences in these parameters between the LAD and other coronary arteries.
Arterial wall characteristics may also help explain atherosclerosis susceptibilities. With regard to the peripheral arteries, although the incidence of atherosclerosis in the radial artery is less in comparison to the coronaries, it is still higher than in the internal mammary artery. While no studies exist comparing the VV density between radial and internal mammary arteries, fewer endothelial fenestrations, lower intercellular junction permeability, a more continuous internal elastic lamina and a less muscular media in comparison to the radial are some of the factors thought to offer the internal mammary artery, protection from atherosclerosis.28
In our study, several potential factors could have affected the calculated VV densities. Atherosclerosis itself promotes neovascularisation and high VV densities obtained in diseased arteries may be the result of response of the arteries to the ongoing atherosclerosis rather then a true refection artery’s VV density, which normally determine its atherosclerosis predisposition. Thus only healthy arterial specimens that showed no microscopic evidence of atherosclerosis were used in the study.
However, studies done on porcine arteries following experimental hypercholesterolemia have shown an increase in the VV density that precedes plaque formation.13 In other studies, neovascularization in the epicardial coronary arteries following experimental hypercholesterolemia even preceded endothelial dysfunction when endothelium dependent arterial vasodilation was tested in response to bradykinin after precontraction with endothelin – 1.29
Thus, one cannot rule out the possibility that specimens which appear apparently healthy on microscopy,could have higher VV density from neovascularization, that in some cases could even precede endothelial dysfunction.There are however studies that suggest that the neovascularisation associated with atherosclerosis may be restricted to the tunica media and that it is less likely to affect the adventitial VV density.11
The arterial segments used to calculate VV density in this study were taken from the origin of the respective arteries. Studies done on LAD have shown an absence of segmental heterogeneity in the VV density.28 However, it is possible that such a segmental heterogeneity may exist in other arteries.
Most cadaveric specimens used in our study are obtained through anonymous donations. As a result, the age of the subjects could not be confirmed for this study. Age having an influence on VV density cannot be ruled out.
Conclusion
Significant differences in the VV density exist between the individual coronary arteries, but they do not positively correlate with the atherosclerosis predisposition of the arteries. We propose that rather than just considering the net VV density, the degree of interaction between areas of high and low VV densities should be considered as a potential factor determining atherosclerosis predisposition. Also considering VV densities alone might not completely explain the atherosclerosis predisposition of certain arteries. Hemodynamic factors and structural characteristics of the vessel wall may also be contributing factors. Future studies could also focus on studying the heterogeneity in the functional characteristics of the endothelium of the VV in the different vascular beds. Also, neovascularization which can precede both plaque formation and endothelial dysfunction, needs to be considered as a potential factor affecting calculated VV densities. Further in vivo studies could be done to corroborate the findings obtained in the current study.
Formatting of funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. However upon submission and approval of the project report, the student is provided with a stipend of INR 10,000 by ICMR.
Conflict of interest
None.
Acknowledgements
This project was undertaken as a part of the short-term studentship program conducted by the Indian Council of Medical Research.
References
Cite this article
TY - JOUR AU - Keerthana Panchagnula AU - Rohini Punja AU - Dhiren Punja AU - Chinmay Suryavanshi PY - 2018 DA - 2018/05/04 TI - A comparative study of vasa vasorum density among coronary arteries JO - Artery Research SP - 36 EP - 42 VL - 22 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2018.04.002 DO - 10.1016/j.artres.2018.04.002 ID - Panchagnula2018 ER -
