Porphyromonas gingivalis vesicles reduce MDA-LDL levels and aortic wall thickness in high fat diet induced atherosclerosis rats
- DOI
- 10.1016/j.artres.2018.05.008How to use a DOI?
- Keywords
- Atherosclerosis; Vaccines; MDA-LDL; Vesicles; Porphyromonas gingivalis
- Abstract
Background: Recently, atherosclerosis-associated disease has been reported simultaneously increased. Whereas, to date, no atherosclerosis vaccine is available. Since the epitope mimicry between malondialdehyde low-density lipoprotein (MDA-LDL) and arginine specific epitope gingipain (Rgp) on the Porphyromonas gingivalis vesicles has been reported, it raises an opportunity to employ the potency of P. gingivalis as an atherosclerosis vaccine.
Objective: To evaluate the potency of P. gingivalis vesicles to prevent atherosclerosis, by assessing MDA-LDL level, visceral fat, body weight, and aortic wall thickness, in rats model.
Methods: Five groups of rats (n = 10 per group), three treatment groups, one positive and negative control group were assigned and adapted with high fat diet for 8 weeks. The treatment groups were injected with P. gingivalis vesicles with and without adjuvant with four booster doses. The level of MDA-LDL serum, visceral fat, body weight, and aortic wall thickness were measured in the end of the course.
Results: Our present study found that decreased in MDA-LDL levels (p = 0.037) and aortic wall thickness (p = 0.016) were observed in rats treated with vesicles and adjuvants, but not with vesicles or adjuvants only, compared to negative control. Moreover, MDA-LDL levels in rats immunized with vesicles and adjuvants were significantly lower than healthy rats. However, body weight (p = 0.329 and visceral fat (p = 0.789) were not significantly different in all treatment groups compared to control.
Conclusions: Immunization with P. gingivalis vesicles and adjuvants significantly reduces MDA-LDL level and aortic wall thickness in rats model.
- Copyright
- © 2018 Association for Research into Arterial Structure and Physiology. Published by Elsevier B.V. All rights reserved.
- Open Access
- This is an open access article distributed under the CC BY-NC license.
Introduction
Atherosclerosis, narrowing of the arteries caused by plaque deposits, contributes to a high number of morbidity and mortality of cardiovascular and cerebrovascular diseases globally.1,2 In 2015, there were 17.7 million deaths associated with cardiovascular diseases and representing 31% of all global deaths.3 Although atherosclerosis management has been established,4 the mortality rates due to atherosclerosis-associated diseases have increased in the last two decades.5 This because the management of atherosclerosis is a complex, involving various aspects.6 Therefore, management with a prevention approach such as vaccination is likely to bring better outcome. Several atherosclerosis vaccines have been studied and each of them has a different target site antigen, such as malondialdehyde low-density lipoprotein (MDA-LDL),7–12 native LDL or copper oxidized-LDL,13–15 and p210.16–19 Of these antigens, MDA-LDL is the most widely studied. MDA-LDL is the most important oxidized LDL (ox-LDL) and considered more atherogenic than LDL.20 A study found that the induction of antibodies against MDA-LDL was associated with reduced atherosclerotic lesion formation and lower serum cholesterol level.10
Recently, the correlation between infectious pathogens and the diseases has been proposed to elicit the potency of vaccination, for example: Streptococcus pneumoniae21 and Salmonella typhimurium22 were shown to have an effect on the decreased risk of atherosclerosis; influenza vaccination were found to be associated with reduced risk of stroke,23 and pneumococcal polysaccharide vaccine had been disclosed beneficial for reduction of acute coronary syndrome risk.24 Periodontitis is an inflammation of the periodontal and supporting structures of the teeth, associated with polymicrobial infections including Porphyromonas gingivalis, one of the major contributors of this disease. Scientific evidence revealed that periodontopathogens such as P. gingivalis during periodontitis have a pivotal role in inducing in the development of atherosclerosis.25 This correlation is probably due to the structure mimicry between MDA-LDL and arginine specific epitope gingipain (Rgp) on the vesicles of P. gingivalis, structures with high immunogenicity.26 Theoretically, two high immunogenic properties having similar fragment size have the potency to generate cross-immunity.27 Based on this, this study sought to evaluate the potency of P. gingivalis vesicles to be an atherosclerosis vaccine in rats model assessed by MDA-LDL, visceral fat, body weight, and aortic wall thickness. The results of this study provide a primary data the potency of P. gingivalis vesicles as atherosclerosis vaccine candidate.
Methods
Animal and P. gingivalis outer membrane vesicles
Male albino wistar rats at eight weeks of age were purchased from Physiology Laboratorium, Brawijaya University. Ten of them were randomly assigned into each study group. All animal protocols in this study were approved by the Ethical Committee of Brawijaya University, Malang, Indonesia and were carried out in strict accordance with the Indonesian law and guidelines on the use of experimental animals. The study was conducted in animal facilities at Biomedical Laboratory, Brawijaya University.
P. gingivalis strain (ATCC® 33277™), kindly provided by Supriyono Hasan from Microbiology Laboratory, Faculty of Dentistry, Airlangga University, was used. The outer membrane vesicles of P. gingivalis vesicles were isolated according to previous study.28 Briefly, after separating from the culture media, the bacterial cells were mixed with 40% ammonium sulfate for two hours and centrifuged at 20,000 g for 40 min. The pellet was suspended with Tris buffer (50 mM, pH 9.5) containing 0.5 mM dithiothreitol (DTT) (ThermoFisher, MA, USA) and was dialyzed overnight. The vesicles were then collected by centrifugation (27,000 g for 40 min) and resuspended in 10 ml of Tris buffer. Finally, the suspension was re-centrifuged at 27,000 g for 40 min and the vesicles were resuspended in 1.5 ml of Tris buffer (pH 7.2) and stored at 40 °C for immunization.
High fat diet
High fat diet (HFD) was adapted from previous study with some modifications.29 Briefly, the animals were fed with HFD consisted of 4% cholesterol, 1% cholic acid, 0.5% thiouracil, 10% pork oil, and 5% duck egg yolk. The diet was provided ad libitum. HFD was given at 10 weeks of age in the negative control group and three treatment groups.
P. gingivalis vesicle challenge
Male wistar rats were divided into five groups: three intervention groups and two control groups. The first intervention group (T1) was given a HFD and injected intraperitoneally with 100 μl P. gingivalis vesicles and adjuvants (antigen to adjuvants ratio was 1:1). The second group (T2), was at a HFD and injected with 100 μl vesicles only while the third group (T3) was at a HFD and injected with adjuvants only. A group of rats (PC) were injected with aquabidest while another group (NC) were given HFD only. A booster dose of P. gingivalis vesicles (100 μl) was administered two weeks following the initial dose and repeated until four times in fortnight interval. During initial and booster dose, complete (CFA) and incomplete Freund adjuvants (IFA) were used, respectively. All rats were euthanized after eight weeks on HFD.10
MDA-LDL measurement
MDA-LDL serum levels were measured using ELISA as described previously with some modifications.30 ELISA plates were coated with the capture antibody for MDA-LDL (Abcam, San Francisco, USA) at 10 μg/ml concentration in bicarbonate buffer (pH 9.6) then the plates were incubated at 4 °C overnight. Coating solution was removed and plates were washed with PBS solution. Protein binding sites in coating wells were blocked overnight at 4 °C with 1% skim milk in PBS with 0,5% Tween 20 (PBS-T). Each serum sample was serially diluted with 1% skim milk in PBS-T, added to the well (100 μl each) and incubated for 90 min at 37 °C. The wells were then incubated for one hour at 37 °C with alkaline phosphatase-conjugate goat anti-mouse antibody (Abcam, San Francisco, USA) at a dilution 1:1000. Subsequently, MDA-LDL was detected by chromogenic development using para-nitrophenyl phosphate as the alkaline phosphatase substrate. MDA-LDL was measured in ELISA Reader with absorbance at OD 405 nm λ.
Measurement of visceral fat and body weight
A digital scales (Ohaus CS200. NJ, USA) was used to measure body weight of rats. While for visceral fat measurements, after euthanized using isoflourine anesthesia, epididymal white adipose depots of rats were separated from the epididymis. The perirenal white adipose depots were then dissected. Finally, dissection was conducted in the intra-abdominal mesenteric adipose depot. Visceral fat mass was determined by weighing the perirenal, mesenteric, and periuterine adipose tissue using a digital scales (Ohaus CS200, NJ. USA).
Measurement of aortic wall thickness
Aortic wall thickness was measured using microscopic histopathology as described previously.31 Briefly, after the aorta tissues were embedded and dissected, the aortic sections were deparaffinized by repeated washing with xylene-ethanol and stained with Mayer Hematoxylin and Eosin. The images were captured using a digital camera mounted on a light microscope (Olympus DP71 Microscope Digital Camera, Tokyo, Japan) and the thickness was identified and quantified using image software (Olympus Stream Image Analysis Software, Tokyo, Japan).
Protein profiling of P. gingivalis vesicle and its immunoreactivity
Protein profiles of vesicles isolation were analyzed using SDS PAGE electrophoresis. Vesicles were loaded on SDS PAGE gel consisting of 6% separating and 4% stacking gels followed by Coomassie brilliant blue staining. Then, the protein were transferred to nitrocellulose membranes for further analysis using western blot to investigate their reactivity to rat sera after immunization with P. gingivalis vesicle. Briefly, the proteins were electrophoretically transferred to nitrocellulose membrane at 4 °C overnight at 40 V and 2 h at 100 V. The membranes were blocked for 1 h at room temperature with 3% gelatin in Tris-buffered saline (500 mmol/L NaCl and 20 mmol/L Tris pH 8.0). The membranes were then incubated with rat sera (1:80 in Tris-buffered saline containing 1% gelatin and 0.05% Tween 20) for 2 h at room temperature. This was followed by 1.5 h incubation at room temperature with alkaline phosphatase-conjugated goat anti mouse immunoglobulin G (IgG) (1:1000) (Abcam, San Francisco, USA).10
Statistical analysis
ANOVA test was used to analyze the significance of the differences among variables. Post hoc Tukey was used to determine the differences between treatment and control groups. p-value less than 0.05 were considered significant. Statistical analyses were performed using Software Statistical Product and Service Solution 18 (SPSS 18, SPSS inc. Chicago, IL, USA).
Results
Protein profile of the P. gingivalis vesicles and its immunoreactivity
To identify immunoreactivity of the vesicles of P. gingivalis, the vesicles were extracted and the protein profiles were characterized using SDS-PAGE analysis. The electrophoresis showed that the vesicles contained predominant protein with molecular mass approximately 40 kDa and 28 kDa (Fig. 1A). Western blot showed that the sera from P. gingivalis vesicle-immunize rats were significant immunoreactive against both 40 kDa and 28 kDa protein band (Fig. 1B).
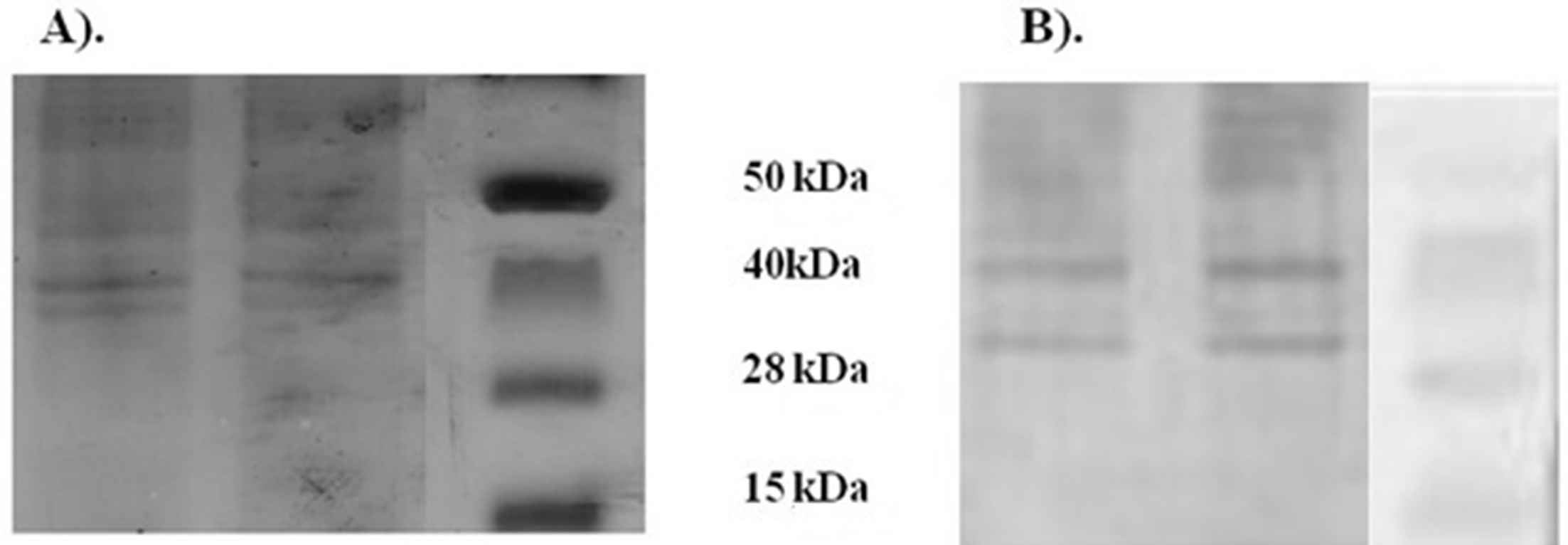
SDS-PAGE and western blot analysis in P. gingivalis vesicles. (A) SDS-PAGE illustrated protein profiles in P. gingivalis vesicles. (B) Western Blot indicated specific binding of serum IgG from rats immunized with P. gingivalis vesicles to the membrane-bound protein component of P. gingivalis vesicles at molecular weight 40 kDa and 28 kDa.
MDA-LDL level
To characterize the specific immune response induced by P. gingivalis vesicles, circulating MDA-LDL were determined by ELISA. Our results showed that MDA-LDL level of PC, T1, T2, T3 and NC group were 24.53 ± 7.65 μg/ml, 22.11 ± 3.59 μg/ml, 36.09 ± 10.28 μg/ml, 42.74 ± 15.71 μg/ml, and 44.73 ± 7.67 μg/ml, respectively. There was significant difference among groups (p = 0.015). The level of MDA-LDL among rats in T1 group was significantly lower compared to NC group (22.11 ± 3.59 μg/ml vs. 44.73 ± 7.67 μg/ml, p = 0.037).
Visceral fat, body weight, and aortic thickness
The mean weight of visceral fats for PC, T1, T2, T3, NC group were 0.98 ± 0.76 g, 2.66 ± 0.37 g, 2.80 ± 0.25 g, 1.93 ± 0.49 g, and 3.13 ± 0.84 g, respectively. There was significant difference among groups (p = 0.001). However, post hoc analysis suggested no significant difference between T1 and NC group (p = 0.789), T2 and NC group (p = 0.929) and T3 and NC group (p = 0.072) (Table 1). Body weights of animal from PC, T1, T2, T33, and NC group were 67.25 ± 19.77 g, 69.75 ± 14.86 g, 100.50 ± 17.02 g, 89.75 ± 9.11 g, and 91.00 ± 13.74 g, respectively. Our analysis using one way ANOVA showed that there was significant difference among groups (p = 0.031). However, post hoc analysis suggested no significant difference between NC and T1 (p = 0.329) or T2 (p = 0.901) or T3 (p = 1.000).
| Parameters | Group (mean ± SD) | p Among groups | p (T1 vs. NC) | p (T2 vs. NC) | p (T3 vs. NC) | p (PC vs. NC) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| PC | T1 | T2 | T3 | NC | ||||||
| MDA-LDL (μg/ml) | 24.53 ± 7.65 | 22.11 ± 3.59 | 36.09 ± 10.28 | 42.74 ± 15.71 | 44.73 ± 7.67 | 0.015 | 0.037 | 0.727 | 0.998 | 0.041 |
| Visceral fat (gram) | 0.98 ± 0.76 | 2.66 ± 0.37 | 2.80 ± 0.25 | 1.93 ± 0.49 | 3.13 ± 0.84 | 0.001 | 0.789 | 0.929 | 0.072 | 0.001 |
| Body weight (gram) | 67.25 ± 19.77 | 69.75 ± 14.86 | 100.50 ± 17.02 | 89.75 ± 9.11 | 91.00 ± 13.74 | 0.031 | 0.329 | 0.901 | 1.000 | 0.235 |
| Aortic thickness (μm) | 26.83 ± 1.39 | 29.01 ± 1.37 | 30.03 ± 0.85 | 31.89 ± 1.23 | 32.91 ± 2.28 | 0.001 | 0.016 | 0.099 | 0.867 | <0.001 |
Note, SD, standard deviation; MDA-LDL, Malondialdehyde low-density lipoprotein; HFD, high fat diet; PC, Positive control (aquabidest); T1, Treatment 1 (HFD + vesicles + adjuvants); T2, Treatment 2 (HFD + vesicles); T3, Treatment 3 (HFD + adjuvants); NC, Negative control (HFD).
Summary of measurements in our study.
As expected, our results showed that the lowest aortic wall was in PC (26.83 ± 1.39 μm) and the highest was in NC (32.91 ± 2.28 μm) and there was significant difference among groups (p = 0.001). However, our data showed that the significant difference was observed only between T1 and NC group, with T1 was lower than NC (29.01 ± 1.37 μm vs. 32.91 ± 2.28 μm, p = 0.016).
Discussions
Although atherosclerosis-associated diseases are reported to increase,32 up to date, vaccine to prevent atherosclerosis is not available. Since P. gingivalis is associated with inflammation and ox-LDL modification,33 this species is considered a potential candidate for atherosclerosis vaccine. Our study was conducted to assess the potency of P. gingivalis to prevent atherosclerosis in rats model by measuring four atherosclerosis indicators: the level of MDA-LDL, visceral fat, body weight, and aortic thickness.
MDA-LDL plays an important role in the development of atherosclerosis.34,35 Several studies had reported that elevated circulating MDA-LDL levels were found in atherosclerosis-associated disease36 including heart disease34,37 and peripheral artery disease.38 Our study found that P. gingivalis vesicles challenge decreased the level of MDA-LDL. This achieved among rats that were treated with both vesicles and adjuvants, but not with vesicle or adjuvant only (Table 1). This suggested that immunization with P. gingivalis vesicles with adjuvant had a protective effect against atherosclerosis, by assessing MD-LDL level, the most important ox-LDL in the pathogenesis of atherosclerosis. The mechanism underlying this association is complex and remains largely undefined. However, this could be explained as follow. MDA-LDL uptake by macrophages would lead to the formation of foam cells and activated foam cells may lead to the upregulation of macrophage receptors, adhesion molecules, the expression of tissue factor, growth factor production, and increase the production of free radicals and inflammatory mediators.39,40 These lead to the thickening of blood vessel walls, defined as atherosclerosis.39,40 There are four possible mechanisms that bridge between P. gingivalis immunization and atherosclerosis. First, Rgp44 on adhesin-hemagglutinin domain of P. gingivalis vesicles are shown to have a high immunogenicity,41 as well as both P1-linear peptide and P2-cyclic peptide on MDA-LDL epitope.42 The mimicry of fragment size between Rgp44, P1-linear peptide, and P2-cyclic peptide allegedly raises a cross-immunity.41,42 As the result, specific antibodies against Rgp44 on the bacterial vesicles may cross-react with peptides on MDA-LDL.26 Second, immunization with P. gingivalis may be able to induce specific IgG serum against atherosclerosis.43 Third, C-reactive protein (CRP) and cytokines may be induced by P. gingivalis immunization.44 Fourth, P. gingivalis immunization may stimulate general activation of B1 cell and interleukin-5 (IL-5) production against atherosclerosis.45 Because of these possible mechanisms, MDA-LDL uptake by macrophages and foam cell formation may be prevented. Moreover, this may also inhibit the adhesion of MDA-LDL in the vascular endothelium. Evidence clearly found that this prevention of foam cells and MDA-LDL accumulation in the intima could ultimately prevent atherosclerosis in vaccinated rats.10,39,42 However, further investigation is required to prove the precise mechanism how P. gingivalis vesicles decrease the MDA-LDL level.
Currently, a number of studies have focused on atherosclerosis vaccine and most studies assumed that MDA-LDL had a great potency to be a target for atherosclerosis vaccine.7–12 Concerning atherosclerosis vaccines with target site at MDA-LDL, our findings confirm and extend the previous studies7–12 that immunizations targeting at MDA-LDL have protective effect against atherosclerosis. Moreover, several studies had reported the potency of P. gingivalis as an atherosclerosis vaccine using various antigens such as 40-kDa outer membrane protein43,44,46,47 and Rgp4448; with different route of administration for example subcutaneous,48 nasal,43,44,46 and oral.47 Although their studies had a very complete and specific design, however, most of them used P. gingivalis to induce atherosclerosis. This leads to the assumption that the vaccines they designed were only effective for atherosclerosis triggered by P. gingivalis. Therefore, the effectiveness of their vaccines against non-infectious atherosclerosis is still questionable. In our present study, we used HFD to induce atherosclerosis. Therefore, it might be assumed that our vaccines had a good effectiveness not only against P. gingivalis-induced atherosclerosis but also non-infectious atherosclerosis. Furthermore, they used only negative controls. Our present study did not only use negative control but also positive control. Therefore, the best and worst data limits could be known. Of those studies, our study is most similar to study by Turunen et al.45 They evaluated MDA-LDL immunization in mice challenged with P. gingivalis and the levels of IgG, IgM, IL-5, IL-10, and interferon-γ (IFN-γ) were measured. They showed that MDA-LDL-immunized mice had elevated IgM and IgG levels to MDA-LDL and increased IL-5 production compared to controls. Overall, they found that MDA-LDL immunization in mice induced with P gingivalis challenge had significantly reduced aortic plaque formation compared to mice without immunization. However, reduction of plaque formation in MDA-LDL-immunized mice was not significant compared to mice that given HFD only, although there were some declines in aortic lipid deposition. Furthermore, the use of LDL modification as atheroprotective immunogen seems to be impractical for generalized use.49 On the other hand, whole bacteria utilization, despite its effectiveness to elicit antibody response against disease, is still controversial following some studies50–53 that linked its infection to accelerate several side effects.
Our results found that freund’s adjuvants, both CFA and IFA alone, had no protective effects against atherosclerosis. Previous studies had shown the protective effect of adjuvants in atherosclerosis.12,54–56 The mechanism of adjuvants as atheroprotective includes their capacity to increase IgG and IgM against MDA-LDL.55,56 Our results were contrast with those previous studies. Further studies are required to elucidate this difference. However, atheroprotective effect of adjuvants is a complex involving several factors. A study revealed that atheroprotective effect of adjuvants is CD4-dependent.12 Therefore, this may explain our results, adjuvants alone may not confer protection against atherosclerosis, and the combination of adjuvants and P. gingivalis vesicles may provide protection against atherosclerosis as reported in our study. However, this reason is not a final. In the near future, we expect there will be studies investigating how the precise mechanism of adjuvants in atherosclerosis.
We also evaluated the effect of P. gingivalis vesicles challenge on aortic wall thickness. Our data indicates that administration of vesicles with adjuvants decrease aortic wall thickness significantly compared to negative control group. This could be because of cross reaction response caused by P. gingivalis immunization. Foam cells accumulation caused by MDA-LDL uptake by macrophages is the basic mechanism for aortic wall thickness and atherosclerosis.39 Therefore, prevention of foam cell accumulation stimulated by P. gingivalis immunization26 has the important role to prevent the thickening of aortic wall. Our study found that P. gingivalis vesicles did not associate with reduction of visceral fat and body weight. One of the plausible reasons is visceral fat and obesity indeed have been linked to atherosclerosis risk factors, however they are chronic and prolonged process.57,58 Therefore, with a short time period of our study, it is understandable that P. gingivalis immunization has no effect on visceral fat and body weight of rats.
Our study demonstrated a good efficacy of P. gingivalis as an atherosclerosis vaccine. Our study was consistent to previous studies concerning P. gingivalis efficacy as an atherosclerosis vaccine in animal models. Further investigation with more specific design or involving genetic approach or in higher level of trials needs to be performed. Moreover, to evaluate the response of society regarding atherosclerosis vaccines, the acceptance and or willingness to pay studies may be needed.
There were several limitations in our study. We did not assess other variable that likely to have effect on atherosclerosis such as the level of triglyceride, cholesterol, fasting blood sugar, CRP, and LDL. In addition, the possibility of a false negative remains due to the small number of tested animals.
Conclusion
Our data suggest that P. gingivalis vesicles have protective effect in preventing atherosclerosis by reducing the level of MDA-LDL and aortic wall thickness in animal model. However, further studies are required to elucidate the mechanisms of these effects.
Author contributions
Designed the experiments = AIM. Performed the experiments = AIM Analyzed the data = AIM, JKF, TH. Contributed reagents/material/analysis tools = AIM. Wrote the manuscript = AIM, JKF, HH, TH. Reference collection and data management = AIM, JKF, HH, TH. Statistical analyses and paper writing = AIM, JKF, HH, TH. Revised manuscript = SRP, EW, MSR, KM, BSP, YP.
Conflict of interest
The authors declared that there is no conflict of interest regarding the publication of this paper.
Appendix A
Supplementary data
Supplementary data related to this article can be found at
References
Cite this article
TY - JOUR AU - Aditya Indra Mahendra AU - Jonny Karunia Fajar AU - Harapan Harapan AU - Teuku Heriansyah AU - Sumarno Reto Prawiro AU - Edi Widjajanto AU - Mohammad Saifur Rohman AU - Karyono Mintaroem AU - Budi Susetio Pikir AU - Yash Prashar PY - 2018 DA - 2018/06/01 TI - Porphyromonas gingivalis vesicles reduce MDA-LDL levels and aortic wall thickness in high fat diet induced atherosclerosis rats JO - Artery Research SP - 20 EP - 27 VL - 23 IS - C SN - 1876-4401 UR - https://doi.org/10.1016/j.artres.2018.05.008 DO - 10.1016/j.artres.2018.05.008 ID - Mahendra2018 ER -
